Multiple dileucine-like motifs direct VGLUT1 trafficking
- PMID: 23804088
- PMCID: PMC3693054
- DOI: 10.1523/JNEUROSCI.5662-12.2013
Multiple dileucine-like motifs direct VGLUT1 trafficking
Abstract
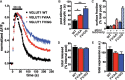
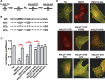
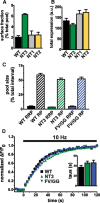
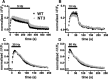
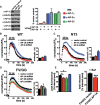
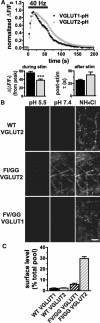
The vesicular glutamate transporters (VGLUTs) package glutamate into synaptic vesicles, and the two principal isoforms VGLUT1 and VGLUT2 have been suggested to influence the properties of release. To understand how a VGLUT isoform might influence transmitter release, we have studied their trafficking and previously identified a dileucine-like endocytic motif in the C terminus of VGLUT1. Disruption of this motif impairs the activity-dependent recycling of VGLUT1, but does not eliminate its endocytosis. We now report the identification of two additional dileucine-like motifs in the N terminus of VGLUT1 that are not well conserved in the other isoforms. In the absence of all three motifs, rat VGLUT1 shows limited accumulation at synaptic sites and no longer responds to stimulation. In addition, shRNA-mediated knockdown of clathrin adaptor proteins AP-1 and AP-2 shows that the C-terminal motif acts largely via AP-2, whereas the N-terminal motifs use AP-1. Without the C-terminal motif, knockdown of AP-1 reduces the proportion of VGLUT1 that responds to stimulation. VGLUT1 thus contains multiple sorting signals that engage distinct trafficking mechanisms. In contrast to VGLUT1, the trafficking of VGLUT2 depends almost entirely on the conserved C-terminal dileucine-like motif: without this motif, a substantial fraction of VGLUT2 redistributes to the plasma membrane and the transporter's synaptic localization is disrupted. Consistent with these differences in trafficking signals, wild-type VGLUT1 and VGLUT2 differ in their response to stimulation.
Figures


Similar articles
-
VGLUT2 Trafficking Is Differentially Regulated by Adaptor Proteins AP-1 and AP-3.Front Cell Neurosci. 2017 Oct 26;11:324. doi: 10.3389/fncel.2017.00324. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29123471 Free PMC article.
-
Protein interactions of the vesicular glutamate transporter VGLUT1.PLoS One. 2014 Oct 15;9(10):e109824. doi: 10.1371/journal.pone.0109824. eCollection 2014. PLoS One. 2014. PMID: 25334008 Free PMC article.
-
Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus.J Neurochem. 2006 Nov;99(3):1011-8. doi: 10.1111/j.1471-4159.2006.04144.x. Epub 2006 Aug 29. J Neurochem. 2006. PMID: 16942593
-
Trafficking of vesicular neurotransmitter transporters.Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review.
-
Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission?Ups J Med Sci. 2010 Feb;115(1):11-20. doi: 10.3109/03009730903572073. Ups J Med Sci. 2010. PMID: 20187846 Free PMC article. Review.
Cited by
-
VGLUT2 Trafficking Is Differentially Regulated by Adaptor Proteins AP-1 and AP-3.Front Cell Neurosci. 2017 Oct 26;11:324. doi: 10.3389/fncel.2017.00324. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29123471 Free PMC article.
-
Control of Synaptotagmin-1 Trafficking by SV2A-Mechanism and Consequences for Presynaptic Function and Dysfunction.J Neurochem. 2025 Jan;169(1):e16308. doi: 10.1111/jnc.16308. J Neurochem. 2025. PMID: 39853744 Free PMC article. Review.
-
Distinct domains of the β1-subunit cytosolic N terminus control surface expression and functional properties of large-conductance calcium-activated potassium (BK) channels.J Biol Chem. 2017 May 26;292(21):8694-8704. doi: 10.1074/jbc.M116.769505. Epub 2017 Apr 3. J Biol Chem. 2017. PMID: 28373283 Free PMC article.
-
Endocytic Adaptor Proteins in Health and Disease: Lessons from Model Organisms and Human Mutations.Cells. 2019 Oct 29;8(11):1345. doi: 10.3390/cells8111345. Cells. 2019. PMID: 31671891 Free PMC article. Review.
-
Presynaptic origins of distinct modes of neurotransmitter release.Curr Opin Neurobiol. 2018 Aug;51:119-126. doi: 10.1016/j.conb.2018.03.005. Epub 2018 Mar 26. Curr Opin Neurobiol. 2018. PMID: 29597140 Free PMC article. Review.
References
-
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–2625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
