A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms
- PMID: 23804097
- PMCID: PMC3693056
- DOI: 10.1523/JNEUROSCI.0735-13.2013
A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms
Abstract
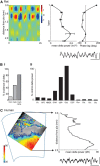
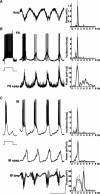
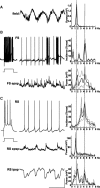
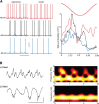
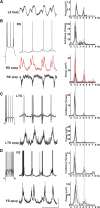
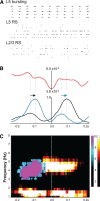
Delta oscillations (1-4 Hz) associate with deep sleep and are implicated in memory consolidation and replay of cortical responses elicited during wake states. A potent local generator has been characterized in thalamus, and local generators in neocortex have been suggested. Here we demonstrate that isolated rat neocortex generates delta rhythms in conditions mimicking the neuromodulatory state during deep sleep (low cholinergic and dopaminergic tone). The rhythm originated in an NMDA receptor-driven network of intrinsic bursting (IB) neurons in layer 5, activating a source of GABAB receptor-mediated inhibition. In contrast, regular spiking (RS) neurons in layer 5 generated theta-frequency outputs. In layer 2/3 principal cells, outputs from IB cells associated with IPSPs, whereas those from layer 5 RS neurons related to nested bursts of theta-frequency EPSPs. Both interlaminar spike and field correlations revealed a sequence of events whereby sparse spiking in layer 2/3 was partially reflected back from layer 5 on each delta period. We suggest that these reciprocal, interlaminar interactions may represent a "Helmholtz machine"-like process to control synaptic rescaling during deep sleep.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
