Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish
- PMID: 23804107
- PMCID: PMC6618491
- DOI: 10.1523/JNEUROSCI.0896-13.2013
Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish
Abstract
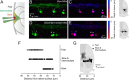
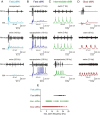
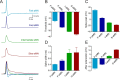
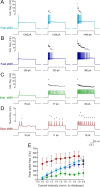
In vertebrates, spinal circuits drive rhythmic firing in motoneurons in the appropriate sequence to produce locomotor movements. These circuits become active early during development and mature gradually to acquire the flexibility necessary to accommodate the increased behavioral repertoire of adult animals. The focus here is to elucidate how different pools of motoneurons are organized and recruited and how membrane properties contribute to their mode of operation. For this purpose, we have used the in vitro preparation of adult zebrafish. We show that different motoneuron pools are organized in a somatotopic fashion in the motor column related to the type of muscle fibers (slow, intermediate, fast) they innervate. During swimming, the different motoneuron pools are recruited in a stepwise manner from slow, to intermediate, to fast to cover the full range of locomotor frequencies seen in intact animals. The spike threshold, filtering properties, and firing patterns of the different motoneuron pools are graded in a manner that relates to their order of recruitment. Our results thus show that motoneurons in adult zebrafish are organized into distinct modules, each with defined locations, properties, and recruitment patterns tuned to precisely match the muscle properties and hence produce swimming of different speeds and modalities.
Figures





References
-
- Bone Q, Kiceniuk J, Jones DR. On the role of the different fibre types in fish myotomes at intermediate swimming speeds. Fishery Bull. 1978;76:691–699.
-
- Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
