Blind evaluation of the microwave-accelerated metal-enhanced fluorescence ultrarapid and sensitive Chlamydia trachomatis test by use of clinical samples
- PMID: 23804384
- PMCID: PMC3754671
- DOI: 10.1128/JCM.00980-13
Blind evaluation of the microwave-accelerated metal-enhanced fluorescence ultrarapid and sensitive Chlamydia trachomatis test by use of clinical samples
Abstract
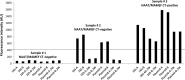
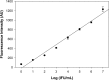
Accurate point-of-care (POC) diagnostic tests for Chlamydia trachomatis infection are urgently needed for the rapid treatment of patients. In a blind comparative study, we evaluated microwave-accelerated metal-enhanced fluorescence (MAMEF) assays for ultrafast and sensitive detection of C. trachomatis DNA from vaginal swabs. The results of two distinct MAMEF assays were compared to those of nucleic acid amplification tests (NAATs). The first assay targeted the C. trachomatis 16S rRNA gene, and the second assay targeted the C. trachomatis cryptic plasmid. Using pure C. trachomatis, the MAMEF assays detected as few as 10 inclusion-forming units/ml of C. trachomatis in less than 9 min, including DNA extraction and detection. A total of 257 dry vaginal swabs from 245 female adolescents aged 14 to 22 years were analyzed. Swabs were eluted with water, the solutions were lysed to release and to fragment genomic DNA, and MAMEF-based DNA detection was performed. The prevalence of C. trachomatis by NAATs was 17.5%. Of the 45 samples that were C. trachomatis positive and the 212 samples that were C. trachomatis negative by NAATs, 33/45 and 197/212 were correctly identified by the MAMEF assays if both assays were required to be positive (sensitivity, 73.3%; specificity, 92.9%). Using the plasmid-based assay alone, 37/45 C. trachomatis-positive and 197/212 C. trachomatis-negative samples were detected (sensitivity, 82.2%; specificity, 92.9%). Using the 16S rRNA assay alone, 34/45 C. trachomatis-positive and 197/212 C. trachomatis-negative samples were detected (sensitivity, 75.5%; specificity, 92.9%). The overall rates of agreement with NAAT results for the individual 16S rRNA and cryptic plasmid assays were 89.5% and 91.0%, respectively. Given the sensitivity, specificity, and rapid detection of the plasmid-based assay, the plasmid-based MAMEF assay appears to be suited for clinical POC testing.
Figures




References
-
- Centers for Disease Control and Prevention 2012. Sexually transmitted disease surveillance, 2011. CDC, Atlanta, GA
-
- Centers for Disease Control and Prevention 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59(RR-12):1–110 - PubMed
-
- National Committee for Quality Assurance 2011. The state of health care quality 2011: HEDIS measures of care, p 77–78 National Committee for Quality Assurance, Washington, DC
-
- Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW, Martin DH, Ferrero DV, Schachter J. 2003. Performance of the APTIMA Combo 2 assay for the multiplex detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J. Clin. Microbiol. 41:304–309 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

