β-mannosylceramide activates type I natural killer t cells to induce tumor immunity without inducing long-term functional anergy
- PMID: 23804426
- PMCID: PMC3819527
- DOI: 10.1158/1078-0432.CCR-12-2169
β-mannosylceramide activates type I natural killer t cells to induce tumor immunity without inducing long-term functional anergy
Abstract
Purpose: Most studies characterizing antitumor properties of invariant natural killer T (iNKT) cells have used the agonist, α-galactosylceramide (α-GalCer). However, α-GalCer induces strong, long-lasting anergy of iNKT cells, which could be a major detriment for clinical therapy. A novel iNKT cell agonist, β-mannosylceramide (β-ManCer), induces strong antitumor immunity through a mechanism distinct from that of α-GalCer. The objective of this study was to determine whether β-ManCer induces anergy of iNKT cells.
Experimental design: Induction of anergy was determined by ex vivo analysis of splenocytes from mice pretreated with iNKT cell agonists as well as in the CT26 lung metastasis in vivo tumor model.
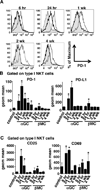
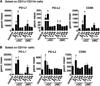
Results: β-ManCer activated iNKT cells without inducing long-term anergy. The transience of anergy induction correlated with a shortened duration of PD-1 upregulation on iNKT cells activated with β-ManCer, compared with α-GalCer. Moreover, whereas mice pretreated with α-GalCer were unable to respond to a second glycolipid stimulation to induce tumor protection for up to 2 months, mice pretreated with β-ManCer were protected from tumors by a second stimulation equivalently to vehicle-treated mice.
Conclusions: The lack of long-term functional anergy induced by β-ManCer, which allows for a second dose to still give therapeutic benefit, suggests the strong potential for this iNKT cell agonist to succeed in settings where α-GalCer has failed.
©2013 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest to be disclosed.
Figures



Similar articles
-
Structure-Function Implications of the Ability of Monoclonal Antibodies Against α-Galactosylceramide-CD1d Complex to Recognize β-Mannosylceramide Presentation by CD1d.Front Immunol. 2019 Oct 9;10:2355. doi: 10.3389/fimmu.2019.02355. eCollection 2019. Front Immunol. 2019. PMID: 31649670 Free PMC article.
-
Serial Stimulation of Invariant Natural Killer T Cells with Covalently Stabilized Bispecific T-cell Engagers Generates Antitumor Immunity While Avoiding Anergy.Cancer Res. 2021 Apr 1;81(7):1788-1801. doi: 10.1158/0008-5472.CAN-20-2219. Epub 2021 Jan 22. Cancer Res. 2021. PMID: 33483371 Free PMC article.
-
Stimulation of a shorter duration in the state of anergy by an invariant natural killer T cell agonist enhances its efficiency of protection from type 1 diabetes.Clin Exp Immunol. 2011 Apr;164(1):26-41. doi: 10.1111/j.1365-2249.2011.04323.x. Epub 2011 Mar 1. Clin Exp Immunol. 2011. PMID: 21361909 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
Cited by
-
PLS-α-GalCer: a novel targeted glycolipid therapy for solid tumors.J Immunother Cancer. 2025 Mar 22;13(3):e009539. doi: 10.1136/jitc-2024-009539. J Immunother Cancer. 2025. PMID: 40121031 Free PMC article.
-
The immunoregulatory role of type I and type II NKT cells in cancer and other diseases.Cancer Immunol Immunother. 2014 Mar;63(3):199-213. doi: 10.1007/s00262-013-1509-4. Epub 2014 Jan 3. Cancer Immunol Immunother. 2014. PMID: 24384834 Free PMC article. Review.
-
Complementary approaches to study NKT cells in cancer.Methods Enzymol. 2020;631:371-389. doi: 10.1016/bs.mie.2019.08.010. Epub 2019 Oct 18. Methods Enzymol. 2020. PMID: 31948558 Free PMC article.
-
Synthetic preparation and immunological evaluation of β-mannosylceramide and related N-acyl analogues.Org Biomol Chem. 2020 Apr 8;18(14):2739-2746. doi: 10.1039/d0ob00223b. Org Biomol Chem. 2020. PMID: 32219267 Free PMC article.
-
NKT cell networks in the regulation of tumor immunity.Front Immunol. 2014 Oct 28;5:543. doi: 10.3389/fimmu.2014.00543. eCollection 2014. Front Immunol. 2014. PMID: 25389427 Free PMC article. Review.
References
-
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. - PubMed
-
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. - PubMed
-
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. - PubMed
-
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. - PubMed
-
- Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

