Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition
- PMID: 23804498
- PMCID: PMC3931734
- DOI: 10.1242/dev.095448
Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition
Abstract
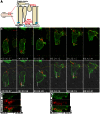
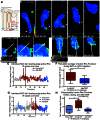
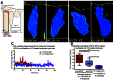
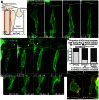
Epithelial-to-mesenchymal transitions (EMTs) are crucial for morphogenesis and carcinoma metastasis, yet mechanisms controlling the underlying cell behaviors are poorly understood. RhoGTPase signaling has been implicated in EMT; however, previous studies have yielded conflicting results regarding Rho function, and its role in EMT remains poorly understood. Elucidation of precise Rho functions has been challenging because Rho signaling is highly context dependent and its activity is tightly regulated spatiotemporally within the cell. To date, few studies have examined how Rho affects cell motility in intact organisms, and the pattern of Rho activity during motile cell behaviors of EMT has not been determined in any system. Here, we image endogenous active Rho during EMT in vivo, and analyze effects of Rho and Rho-kinase (ROCK) manipulation on cell motility in vivo. We show that Rho is activated in a discrete apical region of premigratory neural crest cells during EMT, and Rho-ROCK signaling is essential for apical detachment and generation of motility within the neuroepithelium, a process that has been poorly understood. Furthermore, we find that Arhgap1 restricts Rho activation to apical areas, and this restriction is necessary for detachment. Our results provide new insight into mechanisms controlling local Rho activation and how it affects dynamic cell behaviors and actomyosin contraction during key steps of EMT in an intact living organism.
Keywords: EMT; GAP; Neural crest; RhoGTPase; Zebrafish.
Figures


References
-
- Ahn Y.-H., Gibbons D. L., Chakravarti D., Creighton C. J., Rizvi Z. H., Adams H. P., Pertsemlidis A., Gregory P. A., Wright J. A., Goodall G. J., et al. (2012). ZEB1 drives prometastatic actin cytoskeletal remodeling by downregulating miR-34a expression. J. Clin. Invest. 122, 3170–3183 - PMC - PubMed
-
- Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L., Arteaga C. L. (2000). Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 - PubMed
-
- Barfod E. T., Zheng Y., Kuang W. J., Hart M. J., Evans T., Cerione R. A., Ashkenazi A. (1993). Cloning and expression of a human CDC42 GTPase-activating protein reveals a functional SH3-binding domain. J. Biol. Chem. 268, 26059–26062 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

