Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment
- PMID: 23804507
- PMCID: PMC3704206
- DOI: 10.1177/0272989X13485157
Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment
Abstract
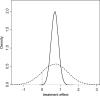
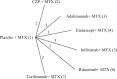
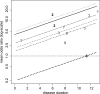
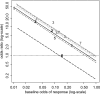
In meta-analysis, between-study heterogeneity indicates the presence of effect-modifiers and has implications for the interpretation of results in cost-effectiveness analysis and decision making. A distinction is usually made between true variability in treatment effects due to variation in patient populations or settings and biases related to the way in which trials were conducted. Variability in relative treatment effects threatens the external validity of trial evidence and limits the ability to generalize from the results; imperfections in trial conduct represent threats to internal validity. We provide guidance on methods for meta-regression and bias-adjustment, in pairwise and network meta-analysis (including indirect comparisons), using illustrative examples. We argue that the predictive distribution of a treatment effect in a "new" trial may, in many cases, be more relevant to decision making than the distribution of the mean effect. Investigators should consider the relative contribution of true variability and random variation due to biases when considering their response to heterogeneity. In network meta-analyses, various types of meta-regression models are possible when trial-level effect-modifying covariates are present or suspected. We argue that a model with a single interaction term is the one most likely to be useful in a decision-making context. Illustrative examples of Bayesian meta-regression against a continuous covariate and meta-regression against "baseline" risk are provided. Annotated WinBUGS code is set out in an appendix.
Keywords: Bayesian meta-analysis; comparative effectiveness; cost-effectiveness analysis; systematic reviews.
Figures
References
-
- National Institute for Health and Clinical Excellence (NICE) Guide to the Methods of Technology Appraisal. London, UK: NICE; 2008 - PubMed
-
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. Chichester, UK: The Cochrane Collaboration, Wiley; 2008
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

