Pharmacologic inhibition of MEK signaling prevents growth of canine hemangiosarcoma
- PMID: 23804705
- PMCID: PMC3769440
- DOI: 10.1158/1535-7163.MCT-12-0893
Pharmacologic inhibition of MEK signaling prevents growth of canine hemangiosarcoma
Abstract
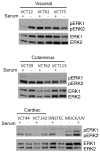
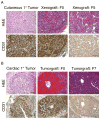
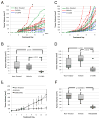
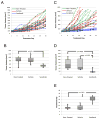
Angiosarcoma is a rare neoplasm of endothelial origin that has limited treatment options and poor five-year survival. As a model for human angiosarcoma, we studied primary cells and tumorgrafts derived from canine hemangiosarcoma (HSA), which is also an endothelial malignancy with similar presentation and histology. Primary cells isolated from HSA showed constitutive extracellular signal-regulated kinase (ERK) activation. The mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitor CI-1040 reduced ERK activation and the viability of primary cells derived from visceral, cutaneous, and cardiac HSA in vitro. HSA-derived primary cells were also sensitive to sorafenib, an inhibitor of B-Raf and multireceptor tyrosine kinases. In vivo, CI-1040 or PD0325901 decreased the growth of cutaneous cell-derived xenografts and cardiac-derived tumorgrafts. Sorafenib decreased tumor size in both in vivo models, although cardiac tumorgrafts were more sensitive. In human angiosarcoma, we noted that 50% of tumors stained positively for phosphorylated ERK1/2 and that the expression of several MEK-responsive transcription factors was upregulated. Our data showed that MEK signaling is essential for the growth of HSA in vitro and in vivo and provided evidence that the same pathways are activated in human angiosarcoma. This indicates that MEK inhibitors may form part of an effective therapeutic strategy for the treatment of canine HSA or human angiosarcoma, and it highlights the use of spontaneous canine cancers as a model of human disease.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


Similar articles
-
Combined inhibition of MEK and mTOR has a synergic effect on angiosarcoma tumorgrafts.Int J Oncol. 2015 Jul;47(1):71-80. doi: 10.3892/ijo.2015.2989. Epub 2015 May 6. Int J Oncol. 2015. PMID: 25955301 Free PMC article.
-
Induction of Bim expression contributes to the antitumor synergy between sorafenib and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor CI-1040 in hepatocellular carcinoma.Clin Cancer Res. 2009 Sep 15;15(18):5820-8. doi: 10.1158/1078-0432.CCR-08-3294. Epub 2009 Sep 8. Clin Cancer Res. 2009. PMID: 19737956
-
Therapeutic potential of MEK inhibition in acute myelogenous leukemia: rationale for "vertical" and "lateral" combination strategies.J Mol Med (Berl). 2012 Oct;90(10):1133-44. doi: 10.1007/s00109-012-0886-z. Epub 2012 Mar 8. J Mol Med (Berl). 2012. PMID: 22399013
-
Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery.Curr Opin Pharmacol. 2005 Aug;5(4):350-6. doi: 10.1016/j.coph.2005.04.007. Curr Opin Pharmacol. 2005. PMID: 15955734 Review.
-
Current understanding of comparative pathology and prospective research approaches for canine hemangiosarcoma.Res Vet Sci. 2024 Feb;167:105120. doi: 10.1016/j.rvsc.2023.105120. Epub 2023 Dec 23. Res Vet Sci. 2024. PMID: 38150941 Review.
Cited by
-
Genomically Complex Human Angiosarcoma and Canine Hemangiosarcoma Establish Convergent Angiogenic Transcriptional Programs Driven by Novel Gene Fusions.Mol Cancer Res. 2021 May;19(5):847-861. doi: 10.1158/1541-7786.MCR-20-0937. Epub 2021 Mar 1. Mol Cancer Res. 2021. PMID: 33649193 Free PMC article.
-
Maintenance therapy with toceranib following doxorubicin-based chemotherapy for canine splenic hemangiosarcoma.BMC Vet Res. 2015 Jun 11;11:131. doi: 10.1186/s12917-015-0446-1. BMC Vet Res. 2015. PMID: 26062540 Free PMC article.
-
Molecular Profile of Canine Hemangiosarcoma and Potential Novel Therapeutic Targets.Vet Sci. 2023 Jun 5;10(6):387. doi: 10.3390/vetsci10060387. Vet Sci. 2023. PMID: 37368773 Free PMC article. Review.
-
tp53 deficiency causes a wide tumor spectrum and increases embryonal rhabdomyosarcoma metastasis in zebrafish.Elife. 2018 Sep 7;7:e37202. doi: 10.7554/eLife.37202. Elife. 2018. PMID: 30192230 Free PMC article.
-
Development of mouse models of angiosarcoma driven by p53.Dis Model Mech. 2019 Jul 9;12(7):dmm038612. doi: 10.1242/dmm.038612. Dis Model Mech. 2019. PMID: 31221668 Free PMC article.
References
-
- Requena L, Sangueza OP. Cutaneous vascular proliferations. Part III. Malignant neoplasms, other cutaneous neoplasms with significant vascular component, and disorders erroneously considered as vascular neoplasms. J Am Acad Dermatol. 1998;38:143–75. - PubMed
-
- Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11:983–91. - PubMed
-
- Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–7. - PubMed
-
- Przygodzki RM, Finkelstein SD, Keohavong P, Zhu D, Bakker A, Swalsky PA, et al. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest. 1997;76:153–9. - PubMed
-
- Marion MJ, Froment O, Trepo C. Activation of Ki-ras gene by point mutation in human liver angiosarcoma associated with vinyl-chloride exposure. Molecular Carcinogenesis. 2005;4:450–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

