Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections
- PMID: 23804714
- PMCID: PMC4907362
- DOI: 10.4049/jimmunol.1300014
Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections
Abstract
Viruses such as influenza suppress host immune function by a variety of methods. This may result in significant morbidity through several pathways, including facilitation of secondary bacterial pneumonia from pathogens such as Streptococcus pneumoniae. PKH26-phagocytic cell labeling dye was administered intranasally to label resident alveolar macrophages (AMs) in a well-established murine model before influenza infection to determine turnover kinetics during the course of infection. More than 90% of resident AMs were lost in the first week after influenza, whereas the remaining cells had a necrotic phenotype. To establish the impact of this innate immune defect, influenza-infected mice were challenged with S. pneumoniae. Early AM-mediated bacterial clearance was significantly impaired in influenza-infected mice: ~50% of the initial bacterial inoculum could be harvested from the alveolar airspace 3 h later. In mock-infected mice, by contrast, >95% of inocula up to 50-fold higher was efficiently cleared. Coinfection during the AM depletion phase caused significant body weight loss and mortality. Two weeks after influenza, the AM population was fully replenished with successful re-establishment of early innate host protection. Local GM-CSF treatment partially restored the impaired early bacterial clearance with efficient protection against secondary pneumococcal pneumonia. We conclude that resident AM depletion occurs during influenza infection. Among other potential effects, this establishes a niche for secondary pneumococcal infection by altering early cellular innate immunity in the lungs, resulting in pneumococcal outgrowth and lethal pneumonia. This novel mechanism will inform development of novel therapeutic approaches to restore lung innate immunity against bacterial superinfections.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
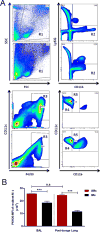
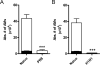





References
-
- Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. - PubMed
-
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. - PubMed
-
- Musher DM, Dowell ME, Shortridge VD, Flamm RK, Jorgensen JH, Le Magueres P, Krause KL. Emergence of macrolide resistance during treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:630–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

