DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice
- PMID: 23804754
- PMCID: PMC3763544
- DOI: 10.1093/nar/gkt556
DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice
Abstract
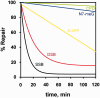
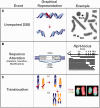
Although the DNA double-strand break (DSB) is defined as a rupture in the double-stranded DNA molecule that can occur without chemical modification in any of the constituent building blocks, it is recognized that this form is restricted to enzyme-induced DSBs. DSBs generated by physical or chemical agents can include at the break site a spectrum of base alterations (lesions). The nature and number of such chemical alterations define the complexity of the DSB and are considered putative determinants for repair pathway choice and the probability that errors will occur during this processing. As the pathways engaged in DSB processing show distinct and frequently inherent propensities for errors, pathway choice also defines the error-levels cells opt to accept. Here, we present a classification of DSBs on the basis of increasing complexity and discuss how complexity may affect processing, as well as how it may cause lethal or carcinogenic processing errors. By critically analyzing the characteristics of DSB repair pathways, we suggest that all repair pathways can in principle remove lesions clustering at the DSB but are likely to fail when they encounter clusters of DSBs that cause a local form of chromothripsis. In the same framework, we also analyze the rational of DSB repair pathway choice.
Figures





References
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd edn. Washington, D.C: ASM Press; 2006.
-
- van Ankeren SC, Murray D, Meyn RE. Induction and rejoining of gamma-ray-induced DNA single- and double-strand breaks in Chinese hamster AA8 cells and in two radiosensitive clones. Radiat. Res. 1988;116:511–525. - PubMed
-
- Nairn RS, Mitchell DL, Adair GM, Thompson LH, Siciliano MJ, Humphrey RM. UV mutagenesis, cytotoxicity and split-dose recovery in a human—CHO cell hybrid having intermediate (6−4) photoproduct repair. Mutat. Res. 1989;217:193–201. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

