Structural basis for S-adenosylmethionine binding and methyltransferase activity by mitochondrial transcription factor B1
- PMID: 23804760
- PMCID: PMC3763538
- DOI: 10.1093/nar/gkt547
Structural basis for S-adenosylmethionine binding and methyltransferase activity by mitochondrial transcription factor B1
Abstract
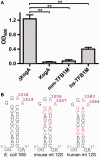
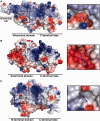
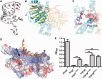
Eukaryotic transcription factor B (TFB) proteins are homologous to KsgA/Dim1 ribosomal RNA (rRNA) methyltransferases. The mammalian TFB1, mitochondrial (TFB1M) factor is an essential protein necessary for mitochondrial gene expression. TFB1M mediates an rRNA modification in the small ribosomal subunit and thus plays a role analogous to KsgA/Dim1 proteins. This modification has been linked to mitochondrial dysfunctions leading to maternally inherited deafness, aminoglycoside sensitivity and diabetes. Here, we present the first structural characterization of the mammalian TFB1 factor. We have solved two X-ray crystallographic structures of TFB1M with (2.1 Å) and without (2.0 Å) its cofactor S-adenosyl-L-methionine. These structures reveal that TFB1M shares a conserved methyltransferase core with other KsgA/Dim1 methyltransferases and shed light on the structural basis of S-adenosyl-L-methionine binding and methyltransferase activity. Together with mutagenesis studies, these data suggest a model for substrate binding and provide insight into the mechanism of methyl transfer, clarifying the role of this factor in an essential process for mitochondrial function.
Figures

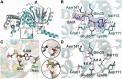

References
-
- Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu. Rev. Biochem. 2007;76:701–722. - PubMed
-
- Zhu X, Peng X, Guan MX, Yan Q. Pathogenic mutations of nuclear genes associated with mitochondrial disorders. Acta Biochim. Biophys. Sin. 2009;41:179–187. - PubMed
-
- Ylikallio E, Suomalainen A. Mechanisms of mitochondrial diseases. Ann. Med. 2012;44:41–59. - PubMed
-
- Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797:113–128. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

