Polyethylene glycol binding alters human telomere G-quadruplex structure by conformational selection
- PMID: 23804761
- PMCID: PMC3763525
- DOI: 10.1093/nar/gkt440
Polyethylene glycol binding alters human telomere G-quadruplex structure by conformational selection
Abstract
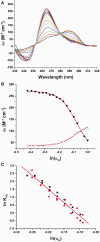
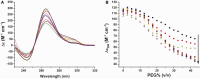
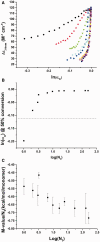
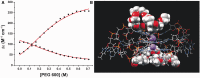
Polyethylene glycols (PEGs) are widely used to perturb the conformations of nucleic acids, including G-quadruplexes. The mechanism by which PEG alters G-quadruplex conformation is poorly understood. We describe here studies designed to determine how PEG and other co-solutes affect the conformation of the human telomeric quadruplex. Osmotic stress studies using acetonitrile and ethylene glycol show that conversion of the 'hybrid' conformation to an all-parallel 'propeller' conformation is accompanied by the release of about 17 water molecules per quadruplex and is energetically unfavorable in pure aqueous solutions. Sedimentation velocity experiments show that the propeller form is hydrodynamically larger than hybrid forms, ruling out a crowding mechanism for the conversion by PEG. PEGs do not alter water activity sufficiently to perturb quadruplex hydration by osmotic stress. PEG titration experiments are most consistent with a conformational selection mechanism in which PEG binds more strongly to the propeller conformation, and binding is coupled to the conformational transition between forms. Molecular dynamics simulations show that PEG binding to the propeller form is sterically feasible and energetically favorable. We conclude that PEG does not act by crowding and is a poor mimic of the intranuclear environment, keeping open the question of the physiologically relevant quadruplex conformation.
Figures
Similar articles
-
G-quadruplexes from human telomeric DNA: how many conformations in PEG containing solutions?J Phys Chem B. 2012 Feb 23;116(7):2294-305. doi: 10.1021/jp209170v. Epub 2012 Feb 8. J Phys Chem B. 2012. PMID: 22268560
-
Molecular engineering of G-quadruplex ligands based on solvent effect of polyethylene glycol.Nucleic Acids Res. 2012 Sep 1;40(17):8711-20. doi: 10.1093/nar/gks578. Epub 2012 Jun 26. Nucleic Acids Res. 2012. PMID: 22735707 Free PMC article.
-
The first derivative of a function of circular dichroism spectra: biophysical study of human telomeric G-quadruplex.Eur Biophys J. 2011 Jan;40(1):29-37. doi: 10.1007/s00249-010-0625-8. Epub 2010 Sep 9. Eur Biophys J. 2011. PMID: 20827473
-
Insights into the free energy landscape and salt-controlled mechanism of the conformational conversions between human telomeric G-quadruplex structures.Int J Biol Macromol. 2021 Nov 30;191:230-242. doi: 10.1016/j.ijbiomac.2021.09.057. Epub 2021 Sep 15. Int J Biol Macromol. 2021. PMID: 34536474
-
Structure of human telomeric DNA in crowded solution.J Am Chem Soc. 2011 Jun 29;133(25):9824-33. doi: 10.1021/ja200786q. Epub 2011 Jun 6. J Am Chem Soc. 2011. PMID: 21548653
Cited by
-
Separating chemical and excluded volume interactions of polyethylene glycols with native proteins: Comparison with PEG effects on DNA helix formation.Biopolymers. 2015 Sep;103(9):517-27. doi: 10.1002/bip.22662. Biopolymers. 2015. PMID: 25924886 Free PMC article.
-
Telomeric G-Quadruplexes: From Human to Tetrahymena Repeats.J Nucleic Acids. 2017;2017:9170371. doi: 10.1155/2017/9170371. Epub 2017 Dec 28. J Nucleic Acids. 2017. PMID: 29445544 Free PMC article.
-
Characterization of human telomere RNA G-quadruplex structures in vitro and in living cells using 19F NMR spectroscopy.Nucleic Acids Res. 2017 May 19;45(9):5501-5511. doi: 10.1093/nar/gkx109. Nucleic Acids Res. 2017. PMID: 28180296 Free PMC article.
-
Zeptoliter DNA Origami Reactor to Reveal Cosolute Effects on Nanoconfined G-Quadruplexes.J Phys Chem Lett. 2022 Sep 22;13(37):8692-8698. doi: 10.1021/acs.jpclett.2c02253. Epub 2022 Sep 12. J Phys Chem Lett. 2022. PMID: 36094396 Free PMC article.
-
Imperfect G-quadruplex as an emerging candidate for transcriptional regulation.Nucleic Acids Res. 2025 Feb 27;53(5):gkaf164. doi: 10.1093/nar/gkaf164. Nucleic Acids Res. 2025. PMID: 40105240 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

