An improved strategy to recover large fragments of functional human neutrophil extracellular traps
- PMID: 23805143
- PMCID: PMC3690357
- DOI: 10.3389/fimmu.2013.00166
An improved strategy to recover large fragments of functional human neutrophil extracellular traps
Abstract
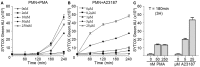
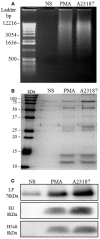
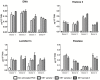
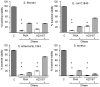
Netosis is a recently described neutrophil function that leads to the release of neutrophil extracellular traps (NETs) in response to various stimuli. NETs are filaments of decondensed chromatin associated with granular proteins. In addition to their role against microorganisms, NETs have been implicated in autoimmunity, thrombosis, and tissue injury. Access to a standardized source of isolated NETs is needed to better analyze the roles of NETs. The aim of this study was to develop a procedure yielding soluble, well-characterized NET preparations from fresh human neutrophils. The calcium ionophore A23187 was chosen to induce netosis, and the restriction enzyme AluI was used to prepare large NET fragments. DNA and proteins were detected by electrophoresis and specific labeling. Some NET proteins [histone 3, lactoferrin (LF)] were quantified by western blotting, and double-stranded DNA (dsDNA) was quantified by immunofluorescence. Co-existence of dsDNA and neutrophil proteins confirmed the quality of the NET preparations. Their biological activity was checked by measuring elastase (ELA) activity and bacterial killing against various strains. Interindividual differences in histone 3, LF, ELA, and dsDNA relative contents were observed in isolated NETs. However, the reproducibility of NET preparation and characterization was validated, suggesting that this interindividual variability was rather related to donor variation than to technical bias. This standardized protocol is suitable for producing, isolating, and quantifying functional NETs that could serve as a tool for studying NET effects on immune cells and tissues.
Keywords: characterization; isolation; microbicidal activity; netosis; neutrophil; neutrophil extracellular traps; quantification.
Figures



References
-
- Betz S. J., Henson P. M. (1980). Production and release of platelet-activating factor (PAF); dissociation from degranulation and superoxide production in the human neutrophil. J. Immunol. 125, 2756–2763 - PubMed
-
- Bianchi M., Niemiec M. J., Siler U., Urban C. F., Reichenbach J. (2011). Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127, e1247.10.1016/j.jaci.2011.01.021 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

