Aberrant Pregnancy Adaptations in the Peripheral Immune Response in Type 1 Diabetes: A Rat Model
- PMID: 23805184
- PMCID: PMC3689741
- DOI: 10.1371/journal.pone.0065490
Aberrant Pregnancy Adaptations in the Peripheral Immune Response in Type 1 Diabetes: A Rat Model
Abstract
Introduction: Despite tight glycemic control, pregnancy complication rate in type 1 diabetes patients is higher than in normal pregnancy. Other etiological factors may be responsible for the development of adverse pregnancy outcome. Acceptance of the semi-allogeneic fetus is accompanied by adaptations in the maternal immune-response. Maladaptations of the immune-response has been shown to contribute to pregnancy complications. We hypothesized that type 1 diabetes, as an autoimmune disease, may be associated with maladaptations of the immune-response to pregnancy, possibly resulting in pregnancy complications.
Methods: We studied pregnancy outcome and pregnancy-induced immunological adaptations in a normoglycemic rat-model of type 1 diabetes, i.e. biobreeding diabetes-prone rats (BBDP; 5 non-pregnant rats, 7 pregnant day 10 rats and 6 pregnant day 18 rats) , versus non-diabetic control rats (i.e. congenic non-diabetic biobreeding diabetes-resistant (BBDR; 6 non-pregnant rats, 6 pregnant day 10 rats and 6 pregnant day 18 rats) and Wistar-rats (6 non-pregnant, 6 pregnant day 10 rats and 5 pregnant day 18 rats)).
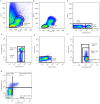
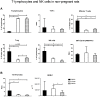
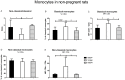
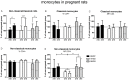
Results: We observed reduced litter size, lower fetal weight of viable fetuses and increased numbers of resorptions versus control rats. These complications are accompanied by various differences in the immune-response between BBDP and control rats in both pregnant and non-pregnant animals. The immune-response in non-pregnant BBDP-rats was characterized by decreased percentages of lymphocytes, increased percentages of effector T-cells, regulatory T-cells and natural killer cells, an increased Th1/Th2-ratio and activated monocytes versus Wistar and BBDR-rats. Furthermore, pregnancy-induced adaptations in BBDP-rats coincided with an increased Th1/Th2-ratio, a decreased mean fluorescence intensity CD161a/NKR-P1b ratio and no further activation of monocytes versus non-diabetic control rats.
Conclusion: This study suggests that even in the face of strict normoglycemia, pregnancy complications still occur in type 1 diabetic pregnancies. This adverse pregnancy outcome may be related to the aberrant immunological adaptations to pregnancy in diabetic rats.
Conflict of interest statement
Figures


References
-
- [Anonymous] (1996) Pregnancy outcomes in the diabetes control and complications trial. Am J Obstet Gynecol 174: 1343–1353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

