The GDNF System Is Altered in Diverticular Disease - Implications for Pathogenesis
- PMID: 23805210
- PMCID: PMC3689736
- DOI: 10.1371/journal.pone.0066290
The GDNF System Is Altered in Diverticular Disease - Implications for Pathogenesis
Abstract
Background & aims: Absence of glial cell line-derived neurotrophic factor (GDNF) leads to intestinal aganglionosis. We recently demonstrated that patients with diverticular disease (DD) exhibit hypoganglionosis suggesting neurotrophic factor deprivation. Thus, we screened mRNA expression pattern of the GDNF system in DD and examined the effects of GDNF on cultured enteric neurons.
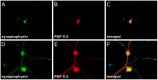
Methods: Colonic specimens obtained from patients with DD (n = 21) and controls (n = 20) were assessed for mRNA expression levels of the GDNF system (GDNF, GDNF receptors GFRα1 and RET). To identify the tissue source of GDNF and its receptors, laser-microdissected (LMD) samples of human myenteric ganglia and intestinal muscle layers were analyzed separately by qPCR. Furthermore, the effects of GDNF treatment on cultured enteric neurons (receptor expression, neuronal differentiation and plasticity) were monitored.
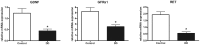
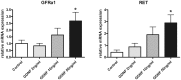
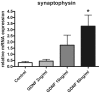
Results: mRNA expression of GDNF and its receptors was significantly down-regulated in the muscularis propria of patients with DD. LMD samples revealed high expression of GDNF in circular and longitudinal muscle layers, whereas GDNF receptors were also expressed in myenteric ganglia. GDNF treatment of cultured enteric neurons increased mRNA expression of its receptors and promoted neuronal differentiation and plasticity revealed by synaptophysin mRNA and protein expression.
Conclusions: Our results suggest that the GDNF system is compromised in DD. In vitro studies demonstrate that GDNF enhances expression of its receptors and promotes enteric neuronal differentiation and plasticity. Since patients with DD exhibit hypoganglionosis, we propose that the observed enteric neuronal loss in DD may be due to lacking neurotrophic support mediated by the GDNF system.
Conflict of interest statement
Figures


References
-
- Jun S, Stollman N (2002) Epidemiology of diverticular disease. Best Pract Res Clin Gastroenterol 16: 529–542. - PubMed
-
- Simpson J, Spiller R (2002) Colonic diverticular disease. Clin Evid: 436–444. - PubMed
-
- Böttner M, Wedel T (2012) Abnormalities of neuromuscular anatomy in diverticular disease. Dig Dis 30: 19–23. - PubMed
-
- Humes DJ, Simpson J, Smith J, Sutton P, Zaitoun A, et al. (2012) Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol Motil 24: 318–e163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

