Mutant Native Outer Membrane Vesicles Combined with a Serogroup A Polysaccharide Conjugate Vaccine for Prevention of Meningococcal Epidemics in Africa
- PMID: 23805230
- PMCID: PMC3689835
- DOI: 10.1371/journal.pone.0066536
Mutant Native Outer Membrane Vesicles Combined with a Serogroup A Polysaccharide Conjugate Vaccine for Prevention of Meningococcal Epidemics in Africa
Abstract
Background: The meningococcal serogroup A (MenA) polysaccharide conjugate vaccine used in Sub-Saharan Africa does not prevent disease caused by MenW or MenX strains, which also cause epidemics in the region. We investigated the vaccine-potential of native outer membrane vesicles with over-expressed factor H-binding protein (NOMV-fHbp), which targeted antigens in African meningococcal strains, and was combined with a MenA polysaccharide conjugate vaccine.
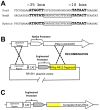
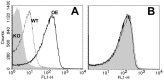
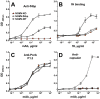
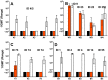
Methodology/principal findings: The NOMV-fHbp vaccine was prepared from a mutant African MenW strain with PorA P1.5,2, attenuated endotoxin (ΔLpxL1), deleted capsular genes, and over-expressed fHbp in variant group 1. The NOMV-fHbp was adsorbed with Al(OH)3 and used to reconstitute a lyophilized MenA conjugate vaccine, which normally is reconstituted with liquid MenC, Y and W conjugates in a meningococcal quadrivalent conjugate vaccine (MCV4-CRM, Novartis). Mice immunized with the NOMV-fHbp vaccine alone developed serum bactericidal (human complement) activity against 13 of 15 African MenA strains tested; 10 of 10 African MenX strains, 7 of 7 African MenW strains, and 6 of 6 genetically diverse MenB strains with fHbp variant group 1 (including 1 strain from The Gambia). The combination NOMV-fHbp/MenA conjugate vaccine elicited high serum bactericidal titers against the two MenA strains tested that were resistant to bactericidal antibodies elicited by the NOMV-fHbp alone; the combination elicited higher titers against the MenA and MenW strains than those elicited by a control MCV4-CRM vaccine (P<0.05); and high titers against MenX and MenB strains. For most strains, the titers elicited by a control NOMV-fHbp knock out vaccine were <1∶10 except when the strain PorA matched the vaccine (titers >1∶000).
Conclusion/significance: The NOMV-fHbp/MenA conjugate vaccine provided similar or higher coverage against MenA and MenW strains than a quadrivalent meningococcal conjugate vaccine, and extended protection against MenX strains responsible for epidemics in Africa, and MenB strains with fHbp in variant group 1.
Conflict of interest statement
Figures



References
-
- Greenwood B (1999) Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 93: 341–353. - PubMed
-
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, et al. (2010) Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 50: 184–191. - PubMed
-
- Harrison LH, Trotter CL, Ramsay ME (2009) Global epidemiology of meningococcal disease. Vaccine 27 Suppl 2B51–63. - PubMed
-
- Jodar L, LaForce FM, Ceccarini C, Aguado T, Granoff DM (2003) Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 361: 1902–1904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

