Formation and analysis of topographical domains between lipid membranes tethered by DNA hybrids of different lengths
- PMID: 23805748
- PMCID: PMC3703934
- DOI: 10.1039/c2fd20108a
Formation and analysis of topographical domains between lipid membranes tethered by DNA hybrids of different lengths
Abstract
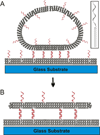
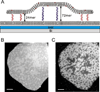
We recently described a strategy to prepare DNA-tethered lipid membranes either to fixed DNA on a surface or to DNA displayed on a supported bilayer [Boxer et al., J. Struct. Biol., 2009, 168, 190; Boxer et al., Langmuir, 2011, 27, 5492]. With the latter system, the DNA hybrids are laterally mobile; when orthogonal sense-antisense pairs of different lengths are used, the DNA hybrids segregate by height and the tethered membrane deforms to accommodate the height difference. This architecture is particularly useful for modelling interactions between membranes mediated by molecular recognition and resembles cell-to-cell junctions. The length, affinity and population of the DNA hybrids between the membranes are completely controllable. Interesting patterns of height segregation are observed by fluorescence interference contrast microscopy. Diverse behavior is observed in the segregation and pattern forming process and possible mechanisms are discussed. This model system captures some of the essential physics of synapse formation and is a step towards understanding lipid membrane behaviour in cell-to-cell junctions.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

