Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial
- PMID: 23805864
- PMCID: PMC3717203
- DOI: 10.1089/jpm.2012.0617
Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial
Abstract
Background: Depression and anxiety are prevalent and undertreated in patients receiving hospice care. Standard antidepressants do not work rapidly or often enough to benefit most of these patients. Ketamine has many properties that make it an interesting candidate for rapidly treating depression and anxiety in patients receiving hospice care. To test this hypothesis, a 28-day, open-label, proof-of-concept trial of daily oral ketamine administration was conducted in order to evaluate the tolerability, potential efficacy, and time to potential efficacy in treating depression and anxiety in patients receiving hospice care.
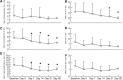
Methods: In this open-label study, 14 subjects with symptoms of depression or depression mixed with anxiety warranting psychopharmacological intervention received daily oral doses of ketamine hydrochloride (0.5 mg/kg) over a 28-day period. The primary outcome measure was the Hospital Anxiety and Depression Scale (HADS), which was used to rate overall depression and anxiety symptoms at baseline, and on days 3, 7, 14, 21, and 28.
Results: Over the 28-day trial there was significant improvement in both depressive symptoms (F5,35=8.03, p=0.002, η(2)=0.534) and symptoms of anxiety (F5,35=14.275, p<0.001, η(2)=0.67) for the eight subjects that completed the trial. One hundred percent of subjects completing the trial responded to ketamine for both anxiety and depression. A significant response in depressive symptoms occurred by day 14 for depression (mean Δ=3.5, d=1.14, 95% CI=1.09-5.9, p=0.01) and day 3 for anxiety (mean Δ=2.4, d=0.67, 95% CI=1.0-3.7, p=0.004). These improvements remained significant through day 28 for both depression (mean Δ=4.0, d=1.34, 95% CI=2.3-5.9, p=0.001) and anxiety (mean Δ=6.09, d=1.34, 95% CI=3.6-8.6, p<0.001). Side effects were rare, the most common being diarrhea, trouble sleeping, and trouble sitting still.
Conclusions: Patients who received daily oral ketamine experienced a robust antidepressant and anxiolytic response with few adverse events. The response rate for depression is similar to those found with IV ketamine; however, the time to response is more protracted. The findings of the potential efficacy of oral ketamine for depression and the response of anxiety symptoms are novel. Further investigation with randomized, controlled clinical trials is necessary to firmly establish the efficacy and safety of oral ketamine for the treatment of depression and anxiety in patients receiving hospice care or other subject populations.
Figures

References
-
- Ferris FD. Balfour HM. Bowen K. Farley J. Hardwick M. Lamontagne C. Lundy M. Syme A. West P. A Model to Guide Hospice Palliative Care. Ottawa, ON: Canadian Hospice Palliative Care Association; 2002. - PubMed
-
- Wilson KG. Chochinov HM. de Faye BJ. Breitbart W. Diagnosis and Management of Depression in Palliative Care. In: Chochinov HM, editor; Breitbart W, editor. Handbook of Psychiatry in Palliative Medicine. New York: Oxford University Press; 2000. pp. 25–50.
-
- NIH: National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J Natl Cancer Inst Monogr. 2004;2004:9–16. - PubMed
-
- Wilson KG. Chochinov HM. Skirko MG. Allard P. Chary S. Gagnon PR. Macmillan K. De Luca M. O'Shea F. Kuhl D. Fainsinger RL. Clinch JJ. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33:118–129. - PubMed
-
- McDaniel JS. Brown FW. Cole SA. Assessment of depression and grief reactions in the medically ill. In: Stoudemire A, editor; Fogel BS, editor; Greenberg DB, editor. Psychiatric Care of the Medical Patient. New York: Oxford University Press; 2000. pp. 149–164.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

