Growth hormone exacerbates diabetic renal damage in male but not female rats
- PMID: 23805912
- PMCID: PMC3698039
- DOI: 10.1186/2042-6410-4-12
Growth hormone exacerbates diabetic renal damage in male but not female rats
Abstract
Background: Human and animal studies support the idea that there are sex differences in the development of diabetic renal disease. Our lab and others have determined that in addition to Ang II (through the AT1R), growth hormone (GH) contributes to renal damage in models of renal failure; however, the impact of sex and GH on the mechanisms initiating diabetic renal disease is not known. This study examined the effect of sex and GH on parameters of renal damage in early, uncontrolled streptozotocin (STZ)-induced diabetes.
Methods: Adult male and female Sprague-Dawley rats were injected with vehicle (control), STZ, or STZ + GH and euthanized after 8 weeks.
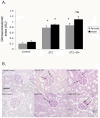
Results: Mild but significant glomerulosclerosis (GS) and tubulointerstitial fibrosis (TIF) was observed in both kidneys from male and female diabetic rats, with GH significantly increasing GS and TIF by 30% and 25% in male rats, but not in female rats. STZ increased TGF-β expression in both kidneys from male and female rats; however, while GH had no further effect on TGF-β protein in diabetic females, GH increased TGF-β protein in the male rat's kidneys by an additional 30%. This sex-specific increase in renal injury following GH treatment was marked by increased MCP-1 and CD-68+ cell density. STZ also reduced renal MMP-2 and MMP-9 protein expression in both kidneys from male and female rats, but additional decreases were only observed in GH-treated diabetic male rats. The sex differences were independent of AT1R activity.
Conclusions: These studies indicate that GH affects renal injury in diabetes in a sex-specific manner and is associated with an increase in pro-inflammatory mediators.
Keywords: Gender; Glomerulosclerosis; Inflammation; Renal Injury; Sex Differences; TGF-β.
Figures





Similar articles
-
Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes.J Endocrinol. 2006 Feb;188(2):263-70. doi: 10.1677/joe.1.06501. J Endocrinol. 2006. PMID: 16461552
-
The accumulation of IGF-I in kidneys of streptozotocin-diabetic adult rats is not associated with elevated plasma GH or IGF-I levels.Endocrine. 1995 Sep;3(9):689-93. doi: 10.1007/BF02746346. Endocrine. 1995. PMID: 21153228
-
Modulation of growth hormone signal transduction in kidneys of streptozotocin-induced diabetic animals: effect of a growth hormone receptor antagonist.Diabetes. 2002 Jul;51(7):2270-81. doi: 10.2337/diabetes.51.7.2270. Diabetes. 2002. PMID: 12086960
-
Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway.J Ethnopharmacol. 2017 Feb 23;198:432-443. doi: 10.1016/j.jep.2016.12.048. Epub 2017 Jan 20. J Ethnopharmacol. 2017. PMID: 28111218
-
[Molecular mechanisms of nephro-protective action of enalapril in experimental chronic renal failure].Ann Acad Med Stetin. 1999;Suppl 52:1-93. Ann Acad Med Stetin. 1999. PMID: 10589103 Review. Polish.
Cited by
-
Novel Actions of Growth Hormone in Podocytes: Implications for Diabetic Nephropathy.Front Med (Lausanne). 2017 Jul 12;4:102. doi: 10.3389/fmed.2017.00102. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28748185 Free PMC article. Review.
-
Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells.Int J Mol Sci. 2017 Aug 12;18(8):1758. doi: 10.3390/ijms18081758. Int J Mol Sci. 2017. PMID: 28805677 Free PMC article.
-
Insight into the Molecular Mechanism of Diabetic Kidney Disease and the Role of Metformin in Its Pathogenesis.Int J Mol Sci. 2023 Aug 22;24(17):13038. doi: 10.3390/ijms241713038. Int J Mol Sci. 2023. PMID: 37685845 Free PMC article. Review.
-
Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria.Cell Death Dis. 2021 Apr 1;12(4):342. doi: 10.1038/s41419-021-03643-6. Cell Death Dis. 2021. PMID: 33795655 Free PMC article.
-
Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy.J Biol Chem. 2019 Nov 1;294(44):16109-16122. doi: 10.1074/jbc.RA119.008966. Epub 2019 Sep 11. J Biol Chem. 2019. PMID: 31511328 Free PMC article.
References
-
- Mok KY, Sandberg K, Sweeny JM, Zheng W, Lee S, Mulroney SE. Growth hormone regulation of glomerular AT1 angiotensin receptors in adult uninephrectomized male rats. Am J Physiol Renal Physiol. 2003;285(6):F1085–F1091. - PubMed
-
- Flyvbjerb A. The role of growth hormone in the pathogenesis of kidney disease. Pediatric Endocrinology Review. 2004;1(Suppl 3):535–539. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

