Mutations in G protein-coupled receptors that impact receptor trafficking and reproductive function
- PMID: 23806559
- PMCID: PMC3844050
- DOI: 10.1016/j.mce.2013.06.024
Mutations in G protein-coupled receptors that impact receptor trafficking and reproductive function
Abstract
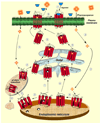
G protein coupled receptors (GPCRs) are a large superfamily of integral cell surface plasma membrane proteins that play key roles in transducing extracellular signals, including sensory stimuli, hormones, neurotransmitters, or paracrine factors into the intracellular environment through the activation of one or more heterotrimeric G proteins. Structural alterations provoked by mutations or variations in the genes coding for GPCRs may lead to misfolding, altered plasma membrane expression of the receptor protein and frequently to disease. A number of GPCRs regulate reproductive function at different levels; these receptors include the gonadotropin-releasing hormone receptor (GnRHR) and the gonadotropin receptors (follicle-stimulating hormone receptor and luteinizing hormone receptor), which regulate the function of the pituitary-gonadal axis. Loss-of-function mutations in these receptors may lead to hypogonadotropic or hypergonadotropic hypogonadism, which encompass a broad spectrum of clinical phenotypes. In this review we describe mutations that provoke misfolding and failure of these receptors to traffick from the endoplasmic reticulum to the plasma membrane. We also discuss some aspects related to the therapeutic potential of some target-specific drugs that selectively bind to and rescue function of misfolded mutant GnRHR and gonadotropin receptors, and that represent potentially valuable strategies to treat diseases caused by inactivating mutations of these receptors.
Keywords: G protein-coupled receptors; Gonadotropin receptors; Gonadotropin-releasing hormone receptor; Hipogonadism; Intracellular trafficking; Pharmacological chaperones.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Misfolded G Protein-Coupled Receptors and Endocrine Disease. Molecular Mechanisms and Therapeutic Prospects.Int J Mol Sci. 2021 Nov 15;22(22):12329. doi: 10.3390/ijms222212329. Int J Mol Sci. 2021. PMID: 34830210 Free PMC article. Review.
-
Restoring function to inactivating G protein-coupled receptor variants in the hypothalamic-pituitary-gonadal axis1.J Neuroendocrinol. 2024 Sep;36(9):e13418. doi: 10.1111/jne.13418. Epub 2024 Jun 9. J Neuroendocrinol. 2024. PMID: 38852954 Review.
-
Pharmacological chaperones correct misfolded GPCRs and rescue function: protein trafficking as a therapeutic target.Subcell Biochem. 2012;63:263-89. doi: 10.1007/978-94-007-4765-4_14. Subcell Biochem. 2012. PMID: 23161143 Review.
-
Intracellular Trafficking of Gonadotropin Receptors in Health and Disease.Handb Exp Pharmacol. 2018;245:1-39. doi: 10.1007/164_2017_49. Handb Exp Pharmacol. 2018. PMID: 29063275 Review.
-
G protein-coupled receptor mutations and human genetic disease.Methods Mol Biol. 2014;1175:153-87. doi: 10.1007/978-1-4939-0956-8_8. Methods Mol Biol. 2014. PMID: 25150870 Review.
Cited by
-
Pharmacological chaperoning: a primer on mechanism and pharmacology.Pharmacol Res. 2014 May;83:10-9. doi: 10.1016/j.phrs.2014.01.005. Epub 2014 Feb 14. Pharmacol Res. 2014. PMID: 24530489 Free PMC article. Review.
-
Reinterpreting anomalous competitive binding experiments within G protein-coupled receptor homodimers using a dimer receptor model.Pharmacol Res. 2019 Jan;139:337-347. doi: 10.1016/j.phrs.2018.11.032. Epub 2018 Nov 22. Pharmacol Res. 2019. PMID: 30472462 Free PMC article.
-
Misfolded G Protein-Coupled Receptors and Endocrine Disease. Molecular Mechanisms and Therapeutic Prospects.Int J Mol Sci. 2021 Nov 15;22(22):12329. doi: 10.3390/ijms222212329. Int J Mol Sci. 2021. PMID: 34830210 Free PMC article. Review.
-
Chaperoning G protein-coupled receptors: from cell biology to therapeutics.Endocr Rev. 2014 Aug;35(4):602-47. doi: 10.1210/er.2013-1121. Epub 2014 Mar 24. Endocr Rev. 2014. PMID: 24661201 Free PMC article. Review.
-
Rescue of mutant gonadotropin-releasing hormone receptor function independent of cognate receptor activity.Sci Rep. 2020 Jun 29;10(1):10579. doi: 10.1038/s41598-020-67473-w. Sci Rep. 2020. PMID: 32601341 Free PMC article.
References
-
- Aittomaki K, Herva R, Stenman UH, Juntunen K, Ylostalo P, Hovatta O, de la Chapelle A. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab. 1996;81:3722–3726. - PubMed
-
- Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de laChapelle A. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. - PubMed
-
- Allen LA, Achermann JC, Pakarinen P, Kotlar TJ, Huhtaniemi IT, Jameson JL, Cheetham TD, Ball SG. A novel loss of function mutation in exon 10 of the FSH receptor gene causing hypergonadotrophic hypogonadism: clinical and molecular characteristics. Hum Reprod. 2003;18:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

