Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo
- PMID: 23806617
- PMCID: PMC4013507
- DOI: 10.1016/j.devcel.2013.05.013
Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo
Abstract
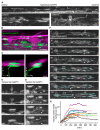
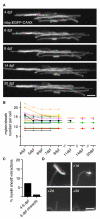
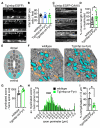
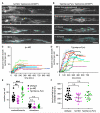
The number of myelin sheaths made by individual oligodendrocytes regulates the extent of myelination, which profoundly affects central nervous system function. It remains unknown when, during their life, individual oligodendrocytes can regulate myelin sheath number in vivo. We show, using live imaging in zebrafish, that oligodendrocytes make new myelin sheaths during a period of just 5 hr, with regulation of sheath number after this time limited to occasional retractions. We also show that activation and reduction of Fyn kinase in oligodendrocytes increases and decreases sheath number per cell, respectively. Interestingly, these oligodendrocytes also generate their new myelin sheaths within the same period, despite having vastly different extents of myelination. Our data demonstrate a restricted time window relative to the lifetime of the individual oligodendrocyte, during which myelin sheath formation occurs and the number of sheaths is determined.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Can't wait to myelinate.Dev Cell. 2013 Jun 24;25(6):549-50. doi: 10.1016/j.devcel.2013.06.003. Dev Cell. 2013. PMID: 23806613
References
-
- Czopka T, Lyons DA. Dissecting mechanisms of myelinated axon formation using zebrafish. Methods Cell Biol. 2011;105:25–62. - PubMed
-
- Dawson MRL, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003;24:476–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

