The Drosophila nuclear lamina protein otefin is required for germline stem cell survival
- PMID: 23806619
- PMCID: PMC3710436
- DOI: 10.1016/j.devcel.2013.05.023
The Drosophila nuclear lamina protein otefin is required for germline stem cell survival
Abstract
LEM domain (LEM-D) proteins are components of an extensive protein network that assembles beneath the inner nuclear envelope. Defects in LEM-D proteins cause tissue-restricted human diseases associated with altered stem cell homeostasis. Otefin (Ote) is a Drosophila LEM-D protein that is intrinsically required for female germline stem cell (GSC) maintenance. Previous studies linked Ote loss with transcriptional activation of the key differentiation gene bag-of-marbles (bam), leading to the model in which Ote tethers the bam gene to the nuclear periphery for gene silencing. Using genetic and phenotypic analyses of multiple ote(-/-) backgrounds, we obtained evidence that is inconsistent with this model. We show that bam repression is maintained in ote(-/-) GSCs and that germ cell loss persists in ote(-/-), bam(-/-) mutants, together demonstrating that GSC loss is independent of bam transcription. We show that the primary defect in ote(-/-) GSCs is a block of differentiation, which ultimately leads to germ cell death.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

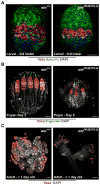
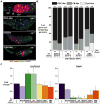
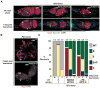
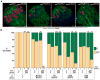
References
-
- Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

