A negative selection methodology using a microfluidic platform for the isolation and enumeration of circulating tumor cells
- PMID: 23806645
- PMCID: PMC3858973
- DOI: 10.1016/j.ymeth.2013.05.027
A negative selection methodology using a microfluidic platform for the isolation and enumeration of circulating tumor cells
Abstract
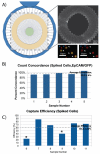
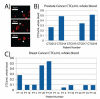
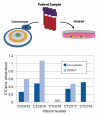
Circulating tumor cells (CTCs) exist in the peripheral blood stream of metastatic cancer patients at rates of approximately 1 CTC per billion background cells. In order to capture and analyze this rare cell population, various techniques exist that range from antibody-based surface marker positive selection to methods that use physical properties of CTCs to negatively exclude background cells from a CTC population. However, methods to capture cells for functional downstream analyses are limited due to inaccessibility of the captured sample or labeling techniques that may be prohibitive to cell function. Here, we present a negative selection method that leverages a Microfluidic Cell Concentrator (MCC) to allow collection and analysis of this rare cell population without needing cell adhesion or other labeling techniques to keep the cells within the chamber. Because the MCC is designed to allow collection and analysis of non-adherent cell populations, multiple staining steps can be applied in parallel to a given CTC population without losing any of the population. The ability of the MCC for patient sample processing of CTCs for enumeration was demonstrated with five patient samples, revealing an average of 0.31 CTCs/mL. The technique was compared to a previously published method - the ELISPOT - that showed similar CTC levels among the five patient samples tested. Because the MCC method does not use positive selection, the method can be applied across a variety of tumor types with no changes to the process.
Keywords: Circulating tumor cells; ELISPOT; Microfluidics; Negative selection.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Nanoroughened adhesion-based capture of circulating tumor cells with heterogeneous expression and metastatic characteristics.BMC Cancer. 2016 Aug 8;16:614. doi: 10.1186/s12885-016-2638-x. BMC Cancer. 2016. PMID: 27501846 Free PMC article.
-
Circulating tumor cells: clinically relevant molecular access based on a novel CTC flow cell.PLoS One. 2014 Jan 29;9(1):e86717. doi: 10.1371/journal.pone.0086717. eCollection 2014. PLoS One. 2014. PMID: 24489774 Free PMC article.
-
Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip.Lab Chip. 2014 Jan 7;14(1):89-98. doi: 10.1039/c3lc51017d. Epub 2013 Nov 13. Lab Chip. 2014. PMID: 24220648 Free PMC article.
-
[Recent advances in isolation and detection of circulating tumor cells with a microfluidic system].Se Pu. 2022 Mar 8;40(3):213-223. doi: 10.3724/SP.J.1123.2021.07009. Se Pu. 2022. PMID: 35243831 Free PMC article. Review. Chinese.
-
Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells.Acc Chem Res. 2014 Oct 21;47(10):2941-50. doi: 10.1021/ar5001617. Epub 2014 Aug 11. Acc Chem Res. 2014. PMID: 25111636 Free PMC article. Review.
Cited by
-
Analytical validation and initial clinical testing of quantitative microscopic evaluation for PD-L1 and HLA I expression on circulating tumor cells from patients with non-small cell lung cancer.Biomark Res. 2022 Apr 25;10(1):26. doi: 10.1186/s40364-022-00370-8. Biomark Res. 2022. PMID: 35468853 Free PMC article.
-
A Stimuli-Responsive, Binary Reagent System for Rapid Isolation of Protein Biomarkers.Anal Chem. 2016 Nov 1;88(21):10404-10410. doi: 10.1021/acs.analchem.6b01961. Epub 2016 Oct 14. Anal Chem. 2016. PMID: 27686335 Free PMC article.
-
Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering.Nat Nanotechnol. 2014 Dec;9(12):969-80. doi: 10.1038/nnano.2014.261. Nat Nanotechnol. 2014. PMID: 25466541 Free PMC article. Review.
-
Clinical Applications of Circulating Tumour Cells and Circulating Tumour DNA in Non-Small Cell Lung Cancer-An Update.Front Oncol. 2022 Mar 15;12:859152. doi: 10.3389/fonc.2022.859152. eCollection 2022. Front Oncol. 2022. PMID: 35372000 Free PMC article. Review.
-
Circulating tumour cells-monitoring treatment response in prostate cancer.Nat Rev Clin Oncol. 2014 Jul;11(7):401-12. doi: 10.1038/nrclinonc.2014.82. Epub 2014 May 13. Nat Rev Clin Oncol. 2014. PMID: 24821215 Review.
References
-
- Mundy GR. Metastasis: Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Reviews Cancer. 2002;2:584–593. - PubMed
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. - PubMed
-
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–42. - PubMed
-
- Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. - PubMed
-
- Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

