Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling
- PMID: 23806960
- PMCID: PMC3695556
- DOI: 10.1038/srep02093
Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling
Abstract
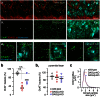
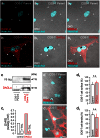
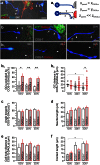
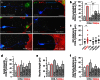
Endocannabinoids are small signaling lipids, with 2-arachidonoylglycerol (2-AG) implicated in modulating axonal growth and synaptic plasticity. The concept of short-range extracellular signaling by endocannabinoids is supported by the lack of trans-synaptic 2-AG signaling in mice lacking sn-1-diacylglycerol lipases (DAGLs), synthesizing 2-AG. Nevertheless, how far endocannabinoids can spread extracellularly to evoke physiological responses at CB₁ cannabinoid receptors (CB₁Rs) remains poorly understood. Here, we first show that cholinergic innervation of CA1 pyramidal cells of the hippocampus is sensitive to the genetic disruption of 2-AG signaling in DAGLα null mice. Next, we exploit a hybrid COS-7-cholinergic neuron co-culture system to demonstrate that heterologous DAGLα overexpression spherically excludes cholinergic growth cones from 2-AG-rich extracellular environments, and minimizes cell-cell contact in vitro. CB₁R-mediated exclusion responses lasted 3 days, indicating sustained spherical 2-AG availability. Overall, these data suggest that extracellular 2-AG concentrations can be sufficient to activate CB₁Rs along discrete spherical boundaries to modulate neuronal responsiveness.
Figures

Similar articles
-
Brain regional cannabinoid CB(1) receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase deficient mice.Eur J Pharm Sci. 2014 Jan 23;51:87-95. doi: 10.1016/j.ejps.2013.08.035. Epub 2013 Sep 3. Eur J Pharm Sci. 2014. PMID: 24012970
-
Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding.J Neurosci. 2010 Oct 20;30(42):13992-4007. doi: 10.1523/JNEUROSCI.2126-10.2010. J Neurosci. 2010. PMID: 20962221 Free PMC article.
-
Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition.Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):26-33. doi: 10.1073/pnas.1522364112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2016. PMID: 26668358 Free PMC article.
-
The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3264-75. doi: 10.1098/rstb.2011.0387. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108545 Free PMC article. Review.
-
Inhibitors of diacylglycerol lipases in neurodegenerative and metabolic disorders.Bioorg Med Chem Lett. 2016 Aug 15;26(16):3831-7. doi: 10.1016/j.bmcl.2016.06.076. Epub 2016 Jun 27. Bioorg Med Chem Lett. 2016. PMID: 27394666 Review.
Cited by
-
Rare genetic variants in the endocannabinoid system genes CNR1 and DAGLA are associated with neurological phenotypes in humans.PLoS One. 2017 Nov 16;12(11):e0187926. doi: 10.1371/journal.pone.0187926. eCollection 2017. PLoS One. 2017. PMID: 29145497 Free PMC article.
-
Neuroprotective Efficacy and Complementary Treatment with Medicinal Herbs: A Comprehensive Review of Recent Therapeutic Approaches in Epilepsy Management.CNS Neurol Disord Drug Targets. 2025;24(1):60-73. doi: 10.2174/0118715273332140240724093837. CNS Neurol Disord Drug Targets. 2025. PMID: 39069797 Review.
-
Endocannabinoid dysfunction in neurological disease: neuro-ocular DAGLA-related syndrome.Brain. 2022 Oct 21;145(10):3383-3390. doi: 10.1093/brain/awac223. Brain. 2022. PMID: 35737950 Free PMC article.
-
Postnatal ethanol exposure alters levels of 2-arachidonylglycerol-metabolizing enzymes and pharmacological inhibition of monoacylglycerol lipase does not cause neurodegeneration in neonatal mice.J Neurochem. 2015 Jul;134(2):276-87. doi: 10.1111/jnc.13120. Epub 2015 Apr 30. J Neurochem. 2015. PMID: 25857698 Free PMC article.
-
Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling.Nat Commun. 2014 Jul 17;5:4421. doi: 10.1038/ncomms5421. Nat Commun. 2014. PMID: 25030704 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

