Molecular pharmacology of store-operated CRAC channels
- PMID: 23807116
- PMCID: PMC3913763
- DOI: 10.4161/chan.25292
Molecular pharmacology of store-operated CRAC channels
Abstract
Calcium influx through store-operated Ca(2+) release-activated Ca(2+) channels (CRAC channels) is a well-defined mechanism of generating cellular Ca(2+) elevations that regulates many functions including gene expression, exocytosis and cell proliferation. The identifications of the ER Ca(2+) sensing proteins, STIM1-2 and the CRAC channel proteins, Orai1-3, have led to improved understanding of the physiological roles and the activation mechanism of CRAC channels. Defects in CRAC channel function are associated with serious human diseases such as immunodeficiency and auto-immunity. In this review, we discuss several pharmacological modulators of CRAC channels, focusing specifically on the molecular mechanism of drug action and their utility in illuminating the mechanism of CRAC channel operation and their physiological roles in different cells.
Keywords: CRAC channel; Orai1; SOCE; STIM1; pharmacology.
Figures

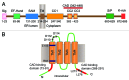
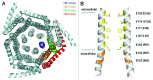
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
