Microfluidic heart on a chip for higher throughput pharmacological studies
- PMID: 23807141
- PMCID: PMC3786400
- DOI: 10.1039/c3lc50350j
Microfluidic heart on a chip for higher throughput pharmacological studies
Abstract
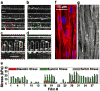
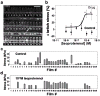
We present the design of a higher throughput "heart on a chip" which utilizes a semi-automated fabrication technique to process sub millimeter sized thin film cantilevers of soft elastomers. Anisotropic cardiac microtissues which recapitulate the laminar architecture of the heart ventricle are engineered on these cantilevers. Deflection of these cantilevers, termed Muscular Thin Films (MTFs), during muscle contraction allows calculation of diastolic and systolic stresses generated by the engineered tissues. We also present the design of a reusable one channel fluidic microdevice completely built out of autoclavable materials which incorporates various features required for an optical cardiac contractility assay: metallic base which fits on a heating element for temperature control, transparent top for recording cantilever deformation and embedded electrodes for electrical field stimulation of the tissue. We employ the microdevice to test the positive inotropic effect of isoproterenol on cardiac contractility at dosages ranging from 1 nM to 100 μM. The higher throughput fluidic heart on a chip has applications in testing of cardiac tissues built from rare or expensive cell sources and for integration with other organ mimics. These advances will help alleviate translational barriers for commercial adoption of these technologies by improving the throughput and reproducibility of readout, standardization of the platform and scalability of manufacture.
Figures


References
-
- Eisenstein EL, Lemons PW, Tardiff BE, Schulman KA, Jolly MK, Califf RM. American Heart Journal. 2005;149:482–488. - PubMed
-
- Fermini B, Fossa AA. Nat Rev Drug Discov. 2003;2:439–447. - PubMed
-
- Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Lab on a Chip. 2012;12:2156–2164. - PubMed
-
- Moraes C, Mehta G, Lesher-Perez SC, Takayama S. Annals of Biomedical Engineering. 2012;40:1211–1227. - PubMed
-
- van der Meer AD, van den Berg A. Integrative Biology. 2012;4:461–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

