Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling
- PMID: 23807172
- PMCID: PMC3721403
- DOI: 10.1038/bjc.2013.334
Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling
Abstract
Background: Sorafenib is a potent inhibitor against Raf kinase and several receptor tyrosine kinases that has been approved for the clinical treatment of advanced renal and liver cancer. Combining sorafenib with other agents has been shown to improve its antitumour efficacy by not only reducing the toxic side effects but also preventing primary and acquired resistance to sorafenib. We have previously observed that tetrandrine exhibits potent antitumour effects in human hepatocellular carcinoma. In this study, we investigated the synergistic antitumour activity of sorafenib in combination with tetrandrine.
Methods: This was a two-part investigation that included the in vitro effects of sorafenib in combination with tetrandrine on cancer cells and the in vivo antitumour efficacy of this drug combination on tumour xenografts in nude mice.
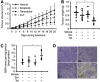
Results: Combined treatment showed a good synergistic antitumour effect yet spared non-tumourigenic cells. The potential molecular mechanism may be mainly that it activated mitochondrial death pathway and induced caspase-dependent apoptosis in the cancer cells. Accumulation of intracellular reactive oxygen species (ROS) and subsequent activation of Akt may also be involved in apoptosis induction.
Conclusion: The antitumour activity of sorafenib plus tetrandrine may be attributed to the induction of the intrinsic apoptosis pathway through ROS/Akt signaling. This finding provides a novel approach that may broaden the clinical application of sorafenib.
Figures





Similar articles
-
ABT-263 enhances sorafenib-induced apoptosis associated with Akt activity and the expression of Bax and p21((CIP1/WAF1)) in human cancer cells.Br J Pharmacol. 2014 Jul;171(13):3182-95. doi: 10.1111/bph.12659. Br J Pharmacol. 2014. PMID: 24571452 Free PMC article.
-
Synergistic anticancer activity of 20(S)-Ginsenoside Rg3 and Sorafenib in hepatocellular carcinoma by modulating PTEN/Akt signaling pathway.Biomed Pharmacother. 2018 Jan;97:1282-1288. doi: 10.1016/j.biopha.2017.11.006. Epub 2017 Dec 14. Biomed Pharmacother. 2018. PMID: 29156516
-
Single Agent and Synergistic Activity of the "First-in-Class" Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma.Mol Cancer Ther. 2016 Nov;15(11):2553-2562. doi: 10.1158/1535-7163.MCT-15-0976. Epub 2016 Aug 5. Mol Cancer Ther. 2016. PMID: 27496136 Free PMC article.
-
Inhibition of MMP-2 Expression Enhances the Antitumor Effect of Sorafenib in Hepatocellular Carcinoma by Suppressing the PI3K/AKT/mTOR Pathway.Oncol Res. 2017 Nov 2;25(9):1543-1553. doi: 10.3727/096504017X14886444100783. Epub 2017 Mar 8. Oncol Res. 2017. PMID: 28276313 Free PMC article.
-
Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma.Int J Cancer. 2011 Sep 15;129(6):1519-31. doi: 10.1002/ijc.25817. Epub 2011 Feb 26. Int J Cancer. 2011. PMID: 21128229
Cited by
-
Synthesis, biological evaluation and toxicity of novel tetrandrine analogues.Eur J Med Chem. 2020 Dec 1;207:112810. doi: 10.1016/j.ejmech.2020.112810. Epub 2020 Sep 4. Eur J Med Chem. 2020. PMID: 32942071 Free PMC article.
-
MHTP, a synthetic tetratetrahydroisoquinoline alkaloid, attenuates lipopolysaccharide-induced acute lung injury via p38MAPK/p65NF-κB signaling pathway-TLR4 dependent.Inflamm Res. 2019 Dec;68(12):1061-1070. doi: 10.1007/s00011-019-01291-3. Epub 2019 Oct 17. Inflamm Res. 2019. PMID: 31624922
-
Melatonin as a regulator of apoptosis in leukaemia: molecular mechanism and therapeutic perspectives.Front Pharmacol. 2023 Aug 14;14:1224151. doi: 10.3389/fphar.2023.1224151. eCollection 2023. Front Pharmacol. 2023. PMID: 37645444 Free PMC article. Review.
-
Tetrandrine inhibits colon carcinoma HT-29 cells growth via the Bcl-2/Caspase 3/PARP pathway and G1/S phase.Biosci Rep. 2019 May 14;39(5):BSR20182109. doi: 10.1042/BSR20182109. Print 2019 May 31. Biosci Rep. 2019. PMID: 31040202 Free PMC article.
-
Anticancer efficacies of arsenic disulfide through apoptosis induction, cell cycle arrest, and pro-survival signal inhibition in human breast cancer cells.Am J Cancer Res. 2018 Mar 1;8(3):366-386. eCollection 2018. Am J Cancer Res. 2018. PMID: 29636995 Free PMC article.
References
-
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303 (5660:1010–1014. - PubMed
-
- Coriat CCR, Chéreau C, Mir O, Alexandre J, Ropert S, Weill B, Chaussade S, Goldwasser F, Batteux F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 2012;11 (10:2284–2293. - PubMed
-
- Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, Nathanson KL, Xia C, Simantov R, Schwartz B, Poulin-Costello M, O'Dwyer PJ, Ratain MJ. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95 (5:581–586. - PMC - PubMed
-
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14 (2:342–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

