Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity
- PMID: 23807209
- PMCID: PMC11113625
- DOI: 10.1007/s00018-013-1404-3
Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity
Abstract
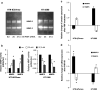
Invasiveness is a common feature of trophoblast and tumors; however, while tumor invasion is uncontrolled, trophoblast invasion is strictly regulated. Both trophoblast and tumor cells express high levels of the immunomodulatory progesterone-induced blocking factor (PIBF), therefore, we aimed to test the possibility that PIBF might be involved in invasion. To this aim, we used PIBF-silenced or PIBF-treated trophoblast (HTR8/Svneo, and primary trophoblast) and tumor (HT-1080, A549, HCT116, PC3) cell lines. Silencing of PIBF increased invasiveness as well as MMP-2,-9 secretion of HTR8/SVneo, and decreased those of HT-1080 cells. PIBF induced immediate STAT6 activation in both cell lines. Silencing of IL-4Rα abrogated all the above effects of PIBF, suggesting that invasion-related signaling by PIBF is initiated through the IL-4Rα/PIBF-receptor complex. In HTR-8/SVneo, PIBF induced fast, but transient Akt and ERK phosphorylation, whereas in tumor cells, PIBF triggered sustained Akt, ERK, and late STAT3 activation. The late signaling events might be due to indirect action of PIBF. PIBF induced the expression of EGF and HB-EGF in HT-1080 cells. The STAT3-activating effect of PIBF was reduced in HB-EGF-deficient HT-1080 cells, suggesting that PIBF-induced HB-EGF contributes to late STAT3 activation. PIBF binds to the promoters of IL-6, EGF, and HB-EGF; however, the protein profile of the protein/DNA complex is different in the two cell lines. We conclude that in tumor cells, PIBF induces proteins, which activate invasion signaling, while-based on our previous data-PIBF might control trophoblast invasion by suppressing proinvasive genes.
Figures






Similar articles
-
Progesterone-induced blocking factor (PIBF) and trophoblast invasiveness.J Reprod Immunol. 2011 Jun;90(1):50-7. doi: 10.1016/j.jri.2011.03.005. Epub 2011 May 31. J Reprod Immunol. 2011. PMID: 21632119
-
The effect of the Progesterone-Induced Blocking Factor (PIBF) on E-cadherin expression, cell motility and invasion of primary tumour cell lines.J Reprod Immunol. 2018 Feb;125:8-15. doi: 10.1016/j.jri.2017.10.047. Epub 2017 Nov 2. J Reprod Immunol. 2018. PMID: 29107859
-
STAT3 and ERK Signaling Pathways Are Implicated in the Invasion Activity by Oncostatin M through Induction of Matrix Metalloproteinases 2 and 9.Yonsei Med J. 2016 May;57(3):761-8. doi: 10.3349/ymj.2016.57.3.761. Yonsei Med J. 2016. PMID: 26996579 Free PMC article.
-
Decreased expression of heparin-binding epidermal growth factor-like growth factor as a newly identified pathogenic mechanism of antiphospholipid-mediated defective placentation.Arthritis Rheum. 2010 May;62(5):1504-12. doi: 10.1002/art.27361. Arthritis Rheum. 2010. PMID: 20131286
-
Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/STAT6 pathway.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt B:463-70. doi: 10.1016/j.jsbmb.2014.09.007. Epub 2014 Sep 12. J Steroid Biochem Mol Biol. 2014. PMID: 25218441
Cited by
-
Progesterone-induced blocking factor 1 and cytokine profile of follicular fluid of infertile women qualified to in vitro fertilization: The influence on fetus development and pregnancy outcome.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221111134. doi: 10.1177/03946320221111134. Int J Immunopathol Pharmacol. 2022. PMID: 35861194 Free PMC article.
-
Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration.Sci Rep. 2016 Jan 27;6:19916. doi: 10.1038/srep19916. Sci Rep. 2016. PMID: 26814137 Free PMC article.
-
A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation.Int J Mol Sci. 2021 Nov 16;22(22):12351. doi: 10.3390/ijms222212351. Int J Mol Sci. 2021. PMID: 34830233 Free PMC article. Review.
-
High Immune Expression of Progesterone-Induced Blocking Factor in Epithelial Ovarian Cancer.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818783911. doi: 10.1177/1533033818783911. Technol Cancer Res Treat. 2018. PMID: 29962287 Free PMC article.
-
Low-Dose Tacrolimus Promotes the Migration and Invasion and Nitric Oxide Production in the Human-Derived First Trimester Extravillous Trophoblast Cells In Vitro.Int J Mol Sci. 2022 Jul 29;23(15):8426. doi: 10.3390/ijms23158426. Int J Mol Sci. 2022. PMID: 35955565 Free PMC article.
References
-
- Szekeres-Bartho J, Kilar F, Falkay G, Csernus V, Torok A, Pacsa AS. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am J Reprod Immunol Microbiol. 1985;9:15–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

