Dual role of chloroquine in liver ischemia reperfusion injury: reduction of liver damage in early phase, but aggravation in late phase
- PMID: 23807223
- PMCID: PMC3702304
- DOI: 10.1038/cddis.2013.225
Dual role of chloroquine in liver ischemia reperfusion injury: reduction of liver damage in early phase, but aggravation in late phase
Abstract
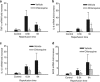
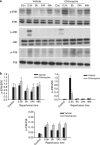
The anti-malaria drug chloroquine is well known as autophagy inhibitor. Chloroquine has also been used as anti-inflammatory drugs to treat inflammatory diseases. We hypothesized that chloroquine could have a dual effect in liver ischemia/reperfusion (I/R) injury: chloroquine on the one hand could protect the liver against I/R injury via inhibition of inflammatory response, but on the other hand could aggravate liver I/R injury through inhibition of autophagy. Rats (n=6 per group) were pre-treated with chloroquine (60 mg/kg, i.p.) 1 h before warm ischemia, and they were continuously subjected to a daily chloroquine injection for up to 2 days. Rats were killed 0.5, 6, 24 and 48 h after reperfusion. At the early phase (i.e., 0-6 h after reperfusion), chloroquine treatment ameliorated liver I/R injury, as indicated by lower serum aminotransferase levels, lower hepatic inflammatory cytokines and fewer histopathologic changes. In contrast, chloroquine worsened liver injury at the late phase of reperfusion (i.e., 24-48 h after reperfusion). The mechanism of protective action of chloroquine appeared to involve its ability to modulate mitogen-activated protein kinase activation, reduce high-mobility group box 1 release and inflammatory cytokines production, whereas chloroquine worsened liver injury via inhibition of autophagy and induction of hepatic apoptosis at the late phase. In conclusion, chloroquine prevents ischemic liver damage at the early phase, but aggravates liver damage at the late phase in liver I/R injury. This dual role of chloroquine should be considered when using chloroquine as an inhibitor of inflammation or autophagy in I/R injury.
Figures




References
-
- Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. - PubMed
-
- Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. - PubMed
-
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

