Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus
- PMID: 23807240
- PMCID: PMC3799073
- DOI: 10.1038/npp.2013.158
Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus
Abstract
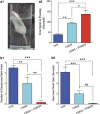
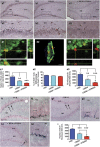
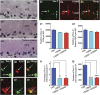
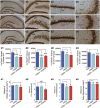
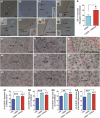
Impairments in mood and cognitive function are the key brain abnormalities observed in Gulf war illness (GWI), a chronic multisymptom health problem afflicting ∼25% of veterans who served in the Persian Gulf War-1. Although the precise cause of GWI is still unknown, combined exposure to a nerve gas prophylaxis drug pyridostigmine bromide (PB) and pesticides DEET and permethrin during the war has been proposed as one of the foremost causes of GWI. We investigated the effect of 4 weeks of exposure to Gulf war illness-related (GWIR) chemicals in the absence or presence of mild stress on mood and cognitive function, dentate gyrus neurogenesis, and neurons, microglia, and astrocytes in the hippocampus. Combined exposure to low doses of GWIR chemicals PB, DEET, and permethrin induced depressive- and anxiety-like behavior and spatial learning and memory dysfunction. Application of mild stress in the period of exposure to chemicals exacerbated the extent of mood and cognitive dysfunction. Furthermore, these behavioral impairments were associated with reduced hippocampal volume and multiple cellular alterations such as chronic reductions in neural stem cell activity and neurogenesis, partial loss of principal neurons, and mild inflammation comprising sporadic occurrence of activated microglia and significant hypertrophy of astrocytes. The results show the first evidence of an association between mood and cognitive dysfunction and hippocampal pathology epitomized by decreased neurogenesis, partial loss of principal neurons, and mild inflammation in a model of GWI. Hence, treatment strategies that are efficacious for enhancing neurogenesis and suppressing inflammation may be helpful for alleviation of mood and cognitive dysfunction observed in GWI.
Figures


References
-
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol Dis. 2002;10:306–326. - PubMed
-
- Abdel-Rahman A, Abou-Donia S, El-Masry E, Shetty A, Abou-Donia M. Stress and combined exposure to low doses of PB, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J Toxicol Environ Health A. 2004a;67:163–192. - PubMed
-
- Abdel-Rahman A, Rao MS, Shetty AK. Nestin expression in hippocampal astrocytes after injury depends on the age. Glia. 2004b;47:299–313. - PubMed
-
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, et al. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Med. 2011;13:275–288. - PubMed
-
- Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, et al. Lipidomic profiling of phosphocholine containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to gulf war agents. Neuromolecular Med. 2012;14:349–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

