Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study
- PMID: 23807273
- PMCID: PMC3694319
- DOI: 10.1097/QAD.0b013e3283612419
Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study
Abstract
Objective: To evaluate the efficacy and safety/tolerability of dolutegravir (DTG, S/GSK1349572), a potent inhibitor of HIV integrase, through the full 96 weeks of the SPRING-1 study.
Design: ING112276 (SPRING-1) was a 96-week, randomized, partially blinded, phase IIb dose-ranging study.
Methods: Treatment-naive adults with HIV received DTG 10, 25, or 50 mg once daily or efavirenz (EFV) 600 mg once daily (control arm) combined with investigator-selected dual nucleos(t)ide reverse transcriptase inhibitor backbone regimen (tenofovir/emtricitabine or abacavir/lamivudine). The primary endpoint of the study was the proportion of participants with plasma HIV-1 RNA less than 50 copies/ml, based on time to loss of virologic response at 16 weeks (conducted for the purpose of phase III dose selection), with a planned analysis at 96 weeks. Safety and tolerability were also assessed.
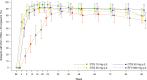
Results: Of 208 participants randomized to treatment, 205 received study drug. At week 96, the proportion of participants achieving plasma HIV-1 RNA less than 50 copies/ml was 79, 78, and 88% for DTG 10, 25, and 50 mg, respectively, compared with 72% for EFV. The median increase from baseline in CD4 cells was 338 cells/μl with DTG (all treatment groups combined) compared with 301 cells/μl with EFV (P = 0.155). No clinically significant dose-related trends in adverse events were observed, and fewer participants who received DTG withdrew because of adverse events (3%) compared with EFV (10%).
Conclusion: Throughout the 96 weeks of the SPRING-1 study, DTG demonstrated sustained efficacy and favorable safety/tolerability in treatment-naive individuals with HIV-1.
Figures
References
-
- Powderly WG. Integrase inhibitors in the treatment of HIV-1 infection. J Antimicrob Chemother 2010; 65:2485–2488 - PubMed
-
- DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. for the 183-0101 Study Team Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr 2006; 43:1–5 - PubMed
-
- Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. for the BENCHMRK Study Teams Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–354 - PubMed
-
- Zolopa AR, Berger DS, Lampiris H, Zhong L, Chuck SL, Enejosa JV, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis 2010; 201:814–822 - PubMed
-
- Lennox JL, DeJesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, et al. for the STARTMRK Investigators Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials