Localization of peripheral autonomic neurons innervating the boar urinary bladder trigone and neurochemical features of the sympathetic component
- PMID: 23807295
- PMCID: PMC3794342
- DOI: 10.4081/ejh.2013.e16
Localization of peripheral autonomic neurons innervating the boar urinary bladder trigone and neurochemical features of the sympathetic component
Abstract
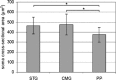
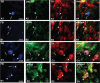
The urinary bladder trigone (UBT) is a limited area through which the majority of vessels and nerve fibers penetrate into the urinary bladder and where nerve fibers and intramural neurons are more concentrated. We localized the extramural post-ganglionic autonomic neurons supplying the porcine UBT by means of retrograde tracing (Fast Blue, FB). Moreover, we investigated the phenotype of sympathetic trunk ganglion (STG) and caudal mesenteric ganglion (CMG) neurons positive to FB (FB+) by coupling retrograde tracing and double-labeling immunofluorescence methods. A mean number of 1845.1±259.3 FB+ neurons were localized bilaterally in the L1-S3 STG, which appeared as small pericarya (465.6±82.7 µm2) mainly localized along an edge of the ganglion. A large number (4287.5±1450.6) of small (476.1±103.9 µm2) FB+ neurons were localized mainly along a border of both CMG. The largest number (4793.3±1990.8) of FB+ neurons was observed in the pelvic plexus (PP), where labeled neurons were often clustered within different microganglia and had smaller soma cross-sectional area (374.9±85.4 µm2). STG and CMG FB+ neurons were immunoreactive (IR) for tyrosine hydroxylase (TH) (66±10.1% and 52.7±8.2%, respectively), dopamine beta-hydroxylase (DβH) (62±6.2% and 52±6.2%, respectively), neuropeptide Y (NPY) (59±8.2% and 65.8±7.3%, respectively), calcitonin-gene-related peptide (CGRP) (24.1±3.3% and 22.1±3.3%, respectively), substance P (SP) (21.6±2.4% and 37.7±7.5%, respectively), vasoactive intestinal polypeptide (VIP) (18.9±2.3% and 35.4±4.4%, respectively), neuronal nitric oxide synthase (nNOS) (15.3±2% and 32.9±7.7%, respectively), vesicular acetylcholine transporter (VAChT) (15±2% and 34.7±4.5%, respectively), leu-enkephalin (LENK) (14.3±7.1% and 25.9±8.9%, respectively), and somatostatin (SOM) (12.4±3% and 31.8±7.3%, respectively). UBT-projecting neurons were also surrounded by VAChT-, CGRP-, LENK-, and nNOS-IR fibers. The possible role of these neurons and fibers in the neural pathways of the UBT is discussed.
Figures






Similar articles
-
Somatostatin immunoreactivity within the urinary bladder nerve fibers and paracervical ganglion urinary bladder projecting neurons in the female pig.J Chem Neuroanat. 2021 Nov;117:102007. doi: 10.1016/j.jchemneu.2021.102007. Epub 2021 Jul 24. J Chem Neuroanat. 2021. PMID: 34314850
-
Localization and neurochemical features of the sympathetic trunk ganglia neurons projecting to the urethral muscle. An experimental study in a porcine animal model.Ann Anat. 2014 Jul;196(4):206-16. doi: 10.1016/j.aanat.2013.12.002. Epub 2014 Jan 18. Ann Anat. 2014. PMID: 24495595
-
Immunohistochemical Properties of the Peripheral Neurons Projecting to the Pig Bulbospongiosus Muscle.Anat Rec (Hoboken). 2016 Sep;299(9):1192-202. doi: 10.1002/ar.23389. Epub 2016 Jul 9. Anat Rec (Hoboken). 2016. PMID: 27342415
-
Autonomic neurons and paraneurons (SIF cells) in the sympathetic ganglia regulating guinea pig proximal colon: immunohistochemical studies.Arch Histol Cytol. 1989;52 Suppl:343-50. doi: 10.1679/aohc.52.suppl_343. Arch Histol Cytol. 1989. PMID: 2510791 Review.
-
The Effect of Castration on Peripheral Autonomic Neurons Supplying Mammalian Male Genitourinary System.Int J Mol Sci. 2021 Jul 16;22(14):7632. doi: 10.3390/ijms22147632. Int J Mol Sci. 2021. PMID: 34299251 Free PMC article. Review.
Cited by
-
A study on preganglionic connections and possible viscerofugal projections from urinary bladder intramural ganglia to the caudal mesenteric ganglion in the pig.J Anat. 2019 Feb;234(2):263-273. doi: 10.1111/joa.12916. Epub 2018 Nov 23. J Anat. 2019. PMID: 30468248 Free PMC article.
-
Detailed Characterization of Sympathetic Chain Ganglia (SChG) Neurons Supplying the Skin of the Porcine Hindlimb.Int J Mol Sci. 2017 Jul 7;18(7):1463. doi: 10.3390/ijms18071463. Int J Mol Sci. 2017. PMID: 28686209 Free PMC article.
-
The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder.Int J Mol Sci. 2021 May 5;22(9):4896. doi: 10.3390/ijms22094896. Int J Mol Sci. 2021. PMID: 34063103 Free PMC article.
-
Anatomical Location of the Vesical Branches of the Inferior Hypogastric Plexus in Human Cadavers.Diagnostics (Basel). 2024 Apr 10;14(8):794. doi: 10.3390/diagnostics14080794. Diagnostics (Basel). 2024. PMID: 38667441 Free PMC article.
-
Changes in the Neurochemical Coding of the Anterior Pelvic Ganglion Neurons Supplying the Male Pig Urinary Bladder Trigone after One-Sided Axotomy of Their Nerve Fibers.Int J Mol Sci. 2021 Feb 24;22(5):2231. doi: 10.3390/ijms22052231. Int J Mol Sci. 2021. PMID: 33668086 Free PMC article.
References
-
- Andersson KE. Bladder activation: afferent mechanisms. Urology 2002;59:43-50 - PubMed
-
- Pidsudko Z. Distribution and chemical coding of neurons in intramural ganglia of the porcine urinary bladder trigone. Folia Histochem Cytobiol 2004;42:3-11 - PubMed
-
- McGaedy TA, Quinn PJ, FitzPatrick ES, Ryan MT. Veterinary embryology, urinary system. Oxford, UK, Blackwell Publishing Ltd., 2006; p 240
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

