Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial
- PMID: 23807371
- PMCID: PMC4162127
- DOI: 10.1001/jamaophthalmol.2013.4154
Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial
Abstract
Importance: The standard care for proliferative diabetic retinopathy (PDR) usually is panretinal photocoagulation, an inherently destructive treatment that can cause iatrogenic vision loss. Therefore, evaluating the effects of therapies for diabetic macular edema on development or worsening of PDR might lead to new therapies for PDR.
Objective: To evaluate the effects of intravitreal ranibizumab or triamcinolone acetonide, administered to treat diabetic macular edema, on worsening of diabetic retinopathy.
Design: Exploratory analysis was performed on worsening of retinopathy, defined as 1 or more of the following: (1) worsening from no PDR to PDR, (2) worsening of 2 or more severity levels on reading center assessment of fundus photographs in eyes without PDR at baseline, (3) having panretinal photocoagulation, (4) experiencing vitreous hemorrhage, or (5) undergoing vitrectomy for the treatment of PDR.
Setting: Community- and university-based ophthalmology practices.
Participants: Individuals with central-involved diabetic macular edema causing visual acuity impairment.
Interventions: Eyes were assigned randomly to sham with prompt focal/grid laser, 0.5 mg of intravitreal ranibizumab with prompt or deferred (≥24 weeks) laser, or 4 mg of intravitreal triamcinolone acetonide with prompt laser.
Main outcomes and measures: Three-year cumulative probabilities for retinopathy worsening.
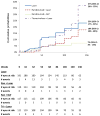
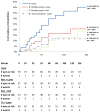
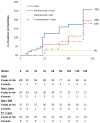
Results: For eyes without PDR at baseline, the 3-year cumulative probabilities for retinopathy worsening (P value comparison with sham with prompt laser) were 23% using sham with prompt laser, 18% with ranibizumab with prompt laser (P = .25), 7% with ranibizumab with deferred laser (P = .001), and 37% with triamcinolone with prompt laser (P = .10). For eyes with PDR at baseline, the 3-year cumulative probabilities for retinopathy worsening were 40%, 21% (P = .05), 18% (P = .02), and 12% (P < .001), respectively. CONCLUSIONS AND RELEVANCE Intravitreal ranibizumab appears to be associated with a reduced risk of diabetic retinopathy worsening in eyes with or without PDR. Intravitreal triamcinolone also appears to be associated with a reduced risk of PDR worsening. These findings suggest that use of these drugs to prevent worsening of diabetic retinopathy may be feasible. Given the exploratory nature of these analyses, the risk of endophthalmitis following intravitreal injections, and the fact that intravitreal triamcinolone can cause cataract or glaucoma, use of these treatments to reduce the rates of worsening of retinopathy, with or without PDR, does not seem warranted at this time.
Figures
References
-
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33. - PubMed
-
- Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583–600. - PubMed
-
- The Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema following focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31:1009–27. - PMC - PubMed
-
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. - PubMed
-
- Oshima Y, Sakaguchi H, Gomi F, et al. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:155–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

