Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study
- PMID: 23807573
- PMCID: PMC3785015
- DOI: 10.1088/0031-9155/58/14/4981
Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study
Abstract
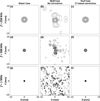
The feasibility of transcranial passive acoustic mapping with hemispherical sparse arrays (30 cm diameter, 16 to 1372 elements, 2.48 mm receiver diameter) using CT-based aberration corrections was investigated via numerical simulations. A multi-layered ray acoustic transcranial ultrasound propagation model based on CT-derived skull morphology was developed. By incorporating skull-specific aberration corrections into a conventional passive beamforming algorithm (Norton and Won 2000 IEEE Trans. Geosci. Remote Sens. 38 1337-43), simulated acoustic source fields representing the emissions from acoustically-stimulated microbubbles were spatially mapped through three digitized human skulls, with the transskull reconstructions closely matching the water-path control images. Image quality was quantified based on main lobe beamwidths, peak sidelobe ratio, and image signal-to-noise ratio. The effects on the resulting image quality of the source's emission frequency and location within the skull cavity, the array sparsity and element configuration, the receiver element sensitivity, and the specific skull morphology were all investigated. The system's resolution capabilities were also estimated for various degrees of array sparsity. Passive imaging of acoustic sources through an intact skull was shown possible with sparse hemispherical imaging arrays. This technique may be useful for the monitoring and control of transcranial focused ultrasound (FUS) treatments, particularly non-thermal, cavitation-mediated applications such as FUS-induced blood-brain barrier disruption or sonothrombolysis, for which no real-time monitoring techniques currently exist.
Figures
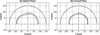









References
-
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford S, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moyé LA, Hill MD, Wojner AW. Ultrasound-enhanced thrombolysis for acute ischemic stroke. New England Journal of Medicine. 2004;14(2):113–117. - PubMed
-
- Aubry J-F, Tanter M, Gerber J, Thomas J-L, Fink M. Optimal focusing by spatio-temporal inverse filter. II. Experiments. Application to focusing through absorbing and reverberating media. Journal of the Acoustical Society of America. 2001;110(1):48–58. - PubMed
-
- Aubry J-F, Tanter M, Pernot M, Thomas J-L, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. Journal of the Acoustical Society of America. 2003;113(1):84–93. - PubMed
-
- Baron C, Aubry J-F, Tanter M, Meairs S, Fink M. Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis. Ultrasound in Medicine & Biology. 2009;35(7):1148–1158. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
