Zinc supplements for treating thalassaemia and sickle cell disease
- PMID: 23807756
- PMCID: PMC9964104
- DOI: 10.1002/14651858.CD009415.pub2
Zinc supplements for treating thalassaemia and sickle cell disease
Abstract
Background: Haemoglobinopathies, inherited disorders of haemoglobin synthesis (thalassaemia) or structure (sickle cell disease), are responsible for significant morbidity and mortality throughout the world. The WHO estimates that, globally, 5% of adults are carriers of a haemoglobin condition, 2.9% are carriers of thalassaemia and 2.3% are carriers of sickle cell disease. Carriers are found worldwide as a result of migration of various ethnic groups to different regions of the world. Zinc is an easily available supplement and intervention programs have been carried out to prevent deficiency in people with thalassaemia or sickle cell anaemia. It is important to evaluate the role of zinc supplementation in the treatment of thalassaemia and sickle cell anaemia to reduce deaths due to complications.
Objectives: To assess the effect of zinc supplementation in the treatment of thalassaemia and sickle cell disease.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.Date of most recent search: 01 February 2013.
Selection criteria: Randomised, placebo-controlled trials of zinc supplements for treating thalassaemia or sickle cell disease administered at least once a week for at least a month.
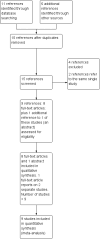
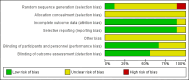
Data collection and analysis: Two review authors assessed the eligibility and risk of bias of the included trials, extracted and analysed data and wrote the review. We summarised results using risk ratios or rate ratios for dichotomous data and mean differences for continuous data. We combined trial results where appropriate.
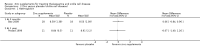
Main results: We identified nine trials for inclusion with all nine contributing outcome data. Two trials reported on people with thalassaemia (n = 152) and seven on sickle cell anaemia (n = 307).In people with thalassaemia, in one trial, the serum zinc level value showed no difference between the zinc supplemented group and the control group, mean difference 47.40 (95% confidence interval -12.95 to 107.99). Regarding anthropometry, in one trial, height velocity was significantly increased in patients who received zinc supplementation for one to seven years duration, mean difference 3.37 (95% confidence interval 2.36 to 4.38) (total number of participants = 26). In one trial, however, there was no difference in body mass index between treatment groups.Zinc acetate supplementation for three months (in one trial) and one year (in two trials) (total number of participants = 71) was noted to increase the serum zinc level significantly in patients with sickle cell anaemia, mean difference 14.90 (95% confidence interval 6.94 to 22.86) and 20.25 (95% confidence interval 11.73 to 28.77) respectively. There was no significant difference in haemoglobin level between intervention and control groups, at either three months (one trial) or one year (one trial), mean difference 0.06 (95% confidence interval -0.84 to 0.96) and mean difference -0.07 (95% confidence interval -1.40 to 1.26) respectively. Regarding anthropometry, one trial showed no significant changes in body mass index or weight after one year of zinc acetate supplementation. In patients with sickle cell disease, the total number of sickle cell crises at one year were significantly decreased in the zinc sulphate supplemented group as compared to controls, mean difference -2.83 (95% confidence interval -3.51 to -2.15) (total participants 130), but not in zinc acetate group, mean difference 1.54 (95% confidence interval -2.01 to 5.09) (total participants 22). In one trial at three months and another at one year, the total number of clinical infections were significantly decreased in the zinc supplemented group as compared to controls, mean difference 0.05 (95% confidence interval 0.01 - 0.43) (total number of participants = 36), and mean difference -7.64 (95% confidence interval -10.89 to -4.39) (total number of participants = 21) respectively.
Authors' conclusions: According to the results, there is no evidence from randomised controlled trials to indicate any benefit of zinc supplementation with regards to serum zinc level in patients with thalassaemia. However, height velocity was noted to increase among those who received this intervention.There is mixed evidence on the benefit of using zinc supplementation in people with sickle cell disease. For instance, there is evidence that zinc supplementation for one year increased the serum zinc levels in patients with sickle cell disease. However, though serum zinc level was raised in patients receiving zinc supplementation, haemoglobin level and anthropometry measurements were not significantly different between groups. Evidence of benefit is seen with the reduction in the number of sickle cell crises among sickle cell patients who received one year of zinc sulphate supplementation and with the reduction in the total number of clinical infections among sickle cell patients who received zinc supplementation for both three months and for one year.The conclusion is based on the data from a small group of trials,which were generally of good quality, with a low risk of bias. The authors recommend that more trials on zinc supplementation in thalassaemia and sickle cell disease be conducted given that the literature has shown the benefits of zinc in these types of diseases.
Conflict of interest statement
None known.
Figures










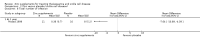
Update of
- doi: 10.1002/14651858.CD009415
References
References to studies included in this review
Acrasoy 1987 {published data only}
-
- Arcasoy A, Cavdar A, Cin S, Erten J, Babacan E, Gözdasoglu S, et al. Effect of zinc supplementation on linear growth in beta thalassaemia. American Journal of Hematology 1987;24(2):127‐36. - PubMed
Bao 2008 {published data only}
-
- Bao B, Prasad A, Beck FWJ, Swerdlow PTI. Beneficial effect of zinc supplementation on oxidative stress, cytokines and NF‐KB DNA binding in sickle cell disease patients [abstract]. Blood 2006;108(11):Abstract number: 1203.
Gupta 1995 {published data only}
-
- Gupta VL, Chaubey BS. Efficacy of zinc therapy in prevention of crisis in sickle cell anemia: a double blind, randomized controlled clinical trial. Journal of the Association of Physicians of India 1995;43(7):467‐9. - PubMed
Prasad 1981 (1) {published data only}
-
- Prasad AS, Abbasi AA, Rabbani P, Dumouchelle E. Effect of zinc supplementation on serum testosterone level in adult male sickle cell anemia subjects. American Journal of Hematology 1981;10(2):119‐27. - PubMed
Prasad 1981 (2) {published data only}
-
- Prasad AS, Abbasi AA, Rabbani P, Dumouchelle E. Effect of zinc supplementation on serum testosterone level in adult male sickle cell anemia subjects. American Journal of Hematology 1981; Vol. 10, issue 2:119‐27. [MEDLINE: ] - PubMed
Prasad 1999 {published data only}
-
- Prasad AS, Beck FW, Kaplan J, Chandrasekar PH, Ortega J, Fitzgerald JT, et al. Effect of zinc supplementation on incidence of infections and hospital admissions in sickle cell disease (SCD). American Journal of Hematology 1999;61(3):194‐202. - PubMed
Rashidi 2011 {published data only}
Serjeant 1970 {published data only}
-
- Serjeant GR, GRE, GMC. Oral zinc sulphate in sickle‐cell ulcers. Lancet 1970; Vol. 2, issue 7679:891‐2. [MEDLINE: ] - PubMed
Zemel 2002 {published data only}
-
- Fung E, Kawchak D, Zemel B, Ohene‐Frempong K, Stallings V. Plasma zinc is not a sensitive indicator of zinc status in children with sickle cell disease [abstract]. Proceedings of the National Sickle Cell Disease Program Annual Meeting; 2001 April 2001:Abstract no: 82.
-
- Zemel BS, Kawchak DA, Fung EB, Ohene‐Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. American Journal of Clinical Nutrition 2002;75(2):300‐7. - PubMed
References to studies excluded from this review
Mehdizadeh 2008 {published data only}
-
- Mehdizadeh M, Zamani G, Tabatabaee S. Zinc status in patients with major beta‐thalassemia. Paediatria Haematology and Oncology 2008;25(1):49‐54. - PubMed
Prasad 1975 {published data only}
-
- Prasad AS, Schoomaker EB, Ortega J, Brewer GJ, Oberleas D, Oelshlegel FJ. Zinc deficiency in sickle cell disease. Clinical Chemistry 1975;21(4):582‐7. - PubMed
Prasad 1983a {published data only}
-
- Prasad AS, Cossack ZT. Zinc in sickle cell disease. Transactions of the Association of Americal Physicians 1983;96:246‐51. - PubMed
Tschumi 1981 {published data only}
-
- Tschumi P, Floersheim GL. Tolerance of large doses of oral zinc sulfate [Zur Vertraglichkeit von hochdosiertem peroralem Zinksulfat.]. Schweizerische Medizinische Wochenschrift 1981; Vol. 111, issue 42:1573‐7. [MEDLINE: ] - PubMed
References to ongoing studies
NCT00459732 {published data only}
-
- Fung EB. Zinc & Bone Health in Thalassemia: The Think Zinc Study (ThinkZn). clinicaltrials.gov (accessed 20 June 2013).
Additional references
Aggett 1995
-
- Aggett PJ, Comerford JG. Zinc and Human Health. Nutrition Reviews 1995;53(9 Pt 2):S16‐S22. - PubMed
Arcasoy 1987
-
- Arcasoy A, Cavdar A, Cin S, Erten J, Babacan E, Gözdasoglu S, et al. Effect of zinc supplementation on linear growth of beta thalassaemia. American Journal of Haematology 1987;24(2):127‐36. - PubMed
Bennekou 2001
-
- Bennekou P, Franceschi L, Pedersen O, Lian L, Asakura T, Evans G, et al. Treatment with NS3623, a novel Cl‐conductance blocker,ameliorates erythrocyte dehydration in transgenic SAD mice: a possible new therapeutic approach for sickle cell disease. Blood 2001;97(5):1451‐7. - PubMed
Bhatangar 2004
-
- Bhatangar S, Natchu UC. Zinc in child health and disease. Indian Journal of Paediatrics 2004;71(11):991‐5. - PubMed
Daeschner 1981
-
- Daeschner CW 3rd, Matustik MC, Carpentieri U, Haggard ME. Zinc and growth in patients with sickle cell disease. Journal of Pediatrics 1981;98(5):778‐80. - PubMed
Firkin 1989
-
- Firkin F, Chesterman C, Peninton D, Rush B. de Gruchy's Clinical Haematology in Medical Practice. 5th Edition. Oxford: Blackwell Scientific Publication, 1989.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Mahyar 2010
Nagalla 2010
Phebus 1988
-
- Phebus CK, Maciak BJ, Gloninger MF, Paul HS. Zinc status of children with sickle cell anaemia: relationship to poor growth. American Journal of Hematology 1988;29(2):67‐73. - PubMed
Prasad 1965
-
- Prasad AS, Diwany M, Gabr M, Sandstead HH, Mokhtar N, Hifney AE. Biochemical studies in thalassemia. Annals of Internal Medicine 1965;62:87‐96. - PubMed
Prasad 1966
-
- Prasad AS. Metabolism of zinc and its deficiency in human subjects. Zinc Metabolism. Springfield: Charles C. Thomas, 1966:250‐303.
Prasad 1983b
-
- Prasad AS. Clinical, Biochemical And Nutritional Spectrum Of Zinc Deficiency In Human Subjects: An Update. Nutrition Reviews 1983;41(7):197‐208. - PubMed
Prasad 1985
-
- Prasad AS. Clinical, endocrinological and biochemical effects of zinc deficiency. Clinics in Endocrinology and Metabolism 1985;14(3):567‐89. - PubMed
Prasad 2007
-
- Prasad AS . The Role of Zinc in Human Health. Academy of Turkish Sciences, 2007.
RDA 1989
-
- Subcommittee on the Tenth Edition of the RDAs of the Food and Nutrition Board. Recommended Dietary Allowances. 10th Edition. Washington DC: National Academy Press, 1989.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Stewart 2007
-
- Stewart KB, EmeryJ, Metcalfe S. Genetics in Family Medicine. 1st Edition. Australia: The Australian Government Agency Biotechnology, 2007.
Thomas 2006
Tracey 2005
-
- Johnston TA. Haemoglobinopathies in pregnancy. The Obstetrician & Gynaecologist 2005;7(3):149‐57.
WHO 1989
-
- World Health Organization. Guidelines for the control of haemoglobin disorders. Report of the VIth Annual Meeting of the WHO working Group on Haemoglobinopathies,Cagliari, Sardinia 8‐9 April 1989.
WHO 2006
-
- WHO Secretariat. Thalassaemia and other haemoglobinopathies. http://apps.who.int/gb/ebwha/pdf_files/EB118/B118_5‐en.pdf (accessed 11 February 2011).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

