Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs
- PMID: 23807770
- PMCID: PMC3928098
- DOI: 10.1038/leu.2013.194
Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs
Abstract
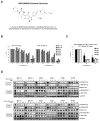
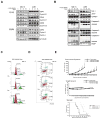
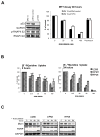
Small-molecule multi-targeted cyclin-dependent kinase (CDK) inhibitors (CDKIs) are of particular interest due to their potent antitumor activity independent of p53 gene alterations. P53 deletion is associated with a very poor prognosis in multiple myeloma (MM). In this regard, we tested the anti-MM activity of RGB-286638, an indenopyrazole-derived CDKI with Ki-nanomolar activity against transcriptional CDKs. We examined RGB-286638's mode-of-action in MM cell lines with wild-type (wt)-p53 and those expressing mutant p53. RGB-286638 treatment resulted in MM cytotoxicity in vitro associated with inhibition of MM tumor growth and prolonged survival in vivo. RGB-286638 displayed caspase-dependent apoptosis in both wt-p53 and mutant-p53 cells that was closely associated with the downregulation of RNA polymerase II phosphorylation and inhibition of transcription. RGB-286638 triggered p53 accumulation via nucleolar stress and loss of Mdm2, accompanied by induction of p53 DNA-binding activity. In addition, RGB-286638 mediated p53-independent activity, which was confirmed by cytotoxicity in p53-knockdown and p53-mutant cells. We also demonstrated downregulation of oncogenic miR-19, miR-92a-1 and miR-21. Our data provide the rationale for the development of transcriptional CDKIs as therapeutic agents, which activate p53 in competent cells, while circumventing p53 deficiency through alternative p53-independent cell death mechanisms in p53-mutant/deleted cells.
Conflict of interest statement
Hannes Loferer is the Agennix AG employee. Other authors have no relevant financial relationship(s) to disclose.
Figures



Similar articles
-
AT7519, A novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3beta activation and RNA polymerase II inhibition.Oncogene. 2010 Apr 22;29(16):2325-36. doi: 10.1038/onc.2009.510. Epub 2010 Jan 25. Oncogene. 2010. PMID: 20101221 Free PMC article.
-
Molecular mechanisms of nutlin-induced apoptosis in multiple myeloma: evidence for p53-transcription-dependent and -independent pathways.Cancer Biol Ther. 2010 Sep 15;10(6):567-78. doi: 10.4161/cbt.10.6.12535. Epub 2010 Oct 1. Cancer Biol Ther. 2010. PMID: 20595817 Free PMC article.
-
Functional p53 in cells contributes to the anticancer effect of the cyclin-dependent kinase inhibitor roscovitine.J Cell Biochem. 2009 Jun 1;107(3):428-37. doi: 10.1002/jcb.22139. J Cell Biochem. 2009. PMID: 19308936
-
Dual action of the inhibitors of cyclin-dependent kinases: targeting of the cell-cycle progression and activation of wild-type p53 protein.Expert Opin Investig Drugs. 2006 Jan;15(1):23-38. doi: 10.1517/13543784.15.1.23. Expert Opin Investig Drugs. 2006. PMID: 16370931 Review.
-
Transcriptional inhibitors, p53 and apoptoss.Biochim Biophys Acta. 2008 Dec;1786(2):83-6. doi: 10.1016/j.bbcan.2008.04.004. Epub 2008 May 8. Biochim Biophys Acta. 2008. PMID: 18503775 Review.
Cited by
-
Novel CDK9 inhibitor oroxylin A promotes wild-type P53 stability and prevents hepatocellular carcinoma progression by disrupting both MDM2 and SIRT1 signaling.Acta Pharmacol Sin. 2022 Apr;43(4):1033-1045. doi: 10.1038/s41401-021-00708-2. Epub 2021 Jun 29. Acta Pharmacol Sin. 2022. PMID: 34188177 Free PMC article.
-
Strategy for drug repurposing in fibroadipogenic replacement during muscle wasting: application to duchenne muscular dystrophy.Front Cell Dev Biol. 2025 Mar 26;13:1505697. doi: 10.3389/fcell.2025.1505697. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40206397 Free PMC article.
-
CDK9 inhibitors in cancer research.RSC Med Chem. 2022 Apr 20;13(6):688-710. doi: 10.1039/d2md00040g. eCollection 2022 Jun 22. RSC Med Chem. 2022. PMID: 35814933 Free PMC article. Review.
-
Cell cycle proteins as promising targets in cancer therapy.Nat Rev Cancer. 2017 Jan 27;17(2):93-115. doi: 10.1038/nrc.2016.138. Nat Rev Cancer. 2017. PMID: 28127048 Free PMC article. Review.
-
MicroRNA-21 and multiple myeloma: small molecule and big function.Med Oncol. 2014 Aug;31(8):94. doi: 10.1007/s12032-014-0094-5. Epub 2014 Jul 1. Med Oncol. 2014. PMID: 24981236 Review.
References
-
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature reviews Cancer. 2009;9(3):153–66. - PubMed
-
- Pinhero R, Liaw P, Bertens K, Yankulov K. Three cyclin-dependent kinases preferentially phosphorylate different parts of the C-terminal domain of the large subunit of RNA polymerase II. European journal of biochemistry/FEBS. 2004;271(5):1004–14. - PubMed
-
- Romano G, Giordano A. Role of the cyclin-dependent kinase 9-related pathway in mammalian gene expression and human diseases. Cell cycle (Georgetown, Tex. 2008;7(23):3664–8. - PubMed
-
- Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23(26):6333–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

