"Designer"-Surfactant-Enabled Cross-Couplings in Water at Room Temperature
- PMID: 23807816
- PMCID: PMC3691853
"Designer"-Surfactant-Enabled Cross-Couplings in Water at Room Temperature
Abstract
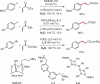
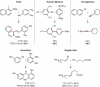
New methodologies are discussed that allow for several commonly used transition-metal-catalyzed coupling reactions to be conducted within aqueous micellar nanoparticles at ambient temperatures.
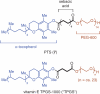
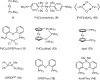
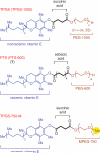
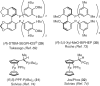
Keywords: PTS; TPGS-750-M; cross-couplings; designer surfactants; green chemistry; micellar catalysis.
Figures








Similar articles
-
Catalysis in the Service of Green Chemistry: Nobel Prize-Winning Palladium-Catalysed Cross-Couplings, Run in Water at Room Temperature: Heck, Suzuki-Miyaura and Negishi reactions carried out in the absence of organic solvents, enabled by micellar catalysis.Platin Met Rev. 2012 Apr;56(2):62-74. doi: 10.1595/147106712x629761. Platin Met Rev. 2012. PMID: 23555153 Free PMC article.
-
On the way towards greener transition-metal-catalyzed processes as quantified by E factors.Angew Chem Int Ed Engl. 2013 Oct 11;52(42):10952-8. doi: 10.1002/anie.201302020. Epub 2013 Sep 12. Angew Chem Int Ed Engl. 2013. PMID: 24030905 Free PMC article. Review.
-
"Nok": a phytosterol-based amphiphile enabling transition-metal-catalyzed couplings in water at room temperature.J Org Chem. 2014 Feb 7;79(3):888-900. doi: 10.1021/jo401744b. Epub 2014 Jan 21. J Org Chem. 2014. PMID: 24447127 Free PMC article.
-
Structure of Nanoparticles Derived from Designer Surfactant TPGS-750-M in Water, As Used in Organic Synthesis.Chemistry. 2018 May 7;24(26):6778-6786. doi: 10.1002/chem.201705524. Epub 2018 Apr 25. Chemistry. 2018. PMID: 29504665
-
Is Micellar Catalysis Green Chemistry?Molecules. 2023 Jun 16;28(12):4809. doi: 10.3390/molecules28124809. Molecules. 2023. PMID: 37375364 Free PMC article. Review.
Cited by
-
Surfactant Micellar and Vesicle Microenvironments and Structures under Synthetic Organic Conditions.J Am Chem Soc. 2023 Apr 5;145(13):7648-7658. doi: 10.1021/jacs.3c01574. Epub 2023 Mar 23. J Am Chem Soc. 2023. PMID: 36951303 Free PMC article.
-
Cascade Processes with Micellar Reaction Media: Recent Advances and Future Directions.Molecules. 2022 Aug 31;27(17):5611. doi: 10.3390/molecules27175611. Molecules. 2022. PMID: 36080376 Free PMC article. Review.
-
Single-Micelle and Single-Zinc-Particle Imaging Provides Insights into the Physical Processes Underpinning Organozinc Reactions in Water.J Am Chem Soc. 2022 Feb 23;144(7):3285-3296. doi: 10.1021/jacs.2c00421. Epub 2022 Feb 14. J Am Chem Soc. 2022. PMID: 35156815 Free PMC article.
-
Sustainable catalysis: rational Pd loading on MIL-101Cr-NH2 for more efficient and recyclable Suzuki-Miyaura reactions.Chemistry. 2013 Dec 16;19(51):17483-93. doi: 10.1002/chem.201302621. Epub 2013 Nov 21. Chemistry. 2013. PMID: 24265270 Free PMC article.
-
Installation of protected ammonia equivalents onto aromatic & heteroaromatic rings in water enabled by micellar catalysis.Green Chem. 2014 Dec 31;16(3):1480-1488. doi: 10.1039/C3GC42188K. Epub 2014 Jan 2. Green Chem. 2014. PMID: 25018667 Free PMC article.
References
-
- [accessed Jan 2012]; http://en.wikipedia.org/wiki/Deepwater_Horizon_oil_spill.
-
- [accessed Jan 2012]; http://bpoilspillcrisisinthegulf.webs.com/corexit.htm.
- [accessed Jan 2012]; http://www.nalco.com/news-and-events/4297.htm.
-
- Zana R, editor. Dynamics of Surfactant Self-Assemblies: Micelles, Microemulsions, Vesicles, and Lyotropic Phases. Taylor & Francis; Boca Raton, FL: 2005. (Surfactant Science Series 125).
- Tadros TF, editor. Applied Surfactants: Principles and Applications. Wiley-VCH; Weinheim, Germany: 2005.
-
- Engberts JBFN. Organic Chemistry in Water: Green and Fast. In: Tundo P, Perosa A, Zecchini F, editors. Methods and Reagents for Green Chemistry: An Introduction. Wiley; Hoboken, NJ: 2007. pp. 159–170. Chapter 7.
-
- Reichardt C, Welton T, editors. Solvents and Solvent Effects in Organic Chemistry. Wiley-VCH; Weinheim, Germany: 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
