Using tablet computers compared to interactive voice response to improve subject recruitment in osteoporosis pragmatic clinical trials: feasibility, satisfaction, and sample size
- PMID: 23807841
- PMCID: PMC3685447
- DOI: 10.2147/PPA.S44551
Using tablet computers compared to interactive voice response to improve subject recruitment in osteoporosis pragmatic clinical trials: feasibility, satisfaction, and sample size
Abstract
Introduction: Pragmatic clinical trials (PCTs) provide large sample sizes and enhanced generalizability to assess therapeutic effectiveness, but efficient patient enrollment procedures are a challenge, especially for community physicians. Advances in technology may improve methods of patient recruitment and screening in PCTs. Our study looked at a tablet computer versus an integrated voice response system (IVRS) for patient recruitment and screening for an osteoporosis PCT in community physician offices.
Materials and methods: We recruited women ≥ 65 years of age from community physician offices to answer screening questions for a hypothetical osteoporosis active comparator PCT using a tablet computer or IVRS. We assessed the feasibility of these technologies for patient recruitment as well as for patient, physician, and office staff satisfaction with the process. We also evaluated the implications of these novel recruitment processes in determining the number of primary care practices and screened patients needed to conduct the proposed trial.
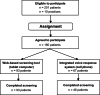
Results: A total of 160 women (80% of those approached) agreed to complete the osteoporosis screening questions in ten family physicians' offices. Women using the tablet computer were able to complete all screening questions consistently and showed a nonsignificant trend towards greater ease of use and willingness to spend more time in their physician's office compared to those using IVRS. Using the proportion of women found to be eligible in this study (almost 20%) and other eligibility scenarios, we determined that between 240 and 670 community physician offices would be needed to recruit ample patients for our hypothetical study.
Conclusion: We found good satisfaction and feasibility with a tablet computer interface for the recruitment and screening of patients for a hypothetical osteoporosis PCT in community office settings. In addition, we used this experience to estimate the number of research sites needed for such a study.
Keywords: clinical trial; computer applications; osteoporosis; pragmatic clinical trials.
Figures
References
-
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. - PubMed
-
- Ware JH, Hamel MB. Pragmatic trials – guides to better patient care? N Engl J Med. 2011;364(18):1685–1687. - PubMed
-
- Prescott RJ, Counsell CE, Gillespie WJ, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3(20):1–143. - PubMed
-
- Black DM, Delmas PD, Eastell R, et al. HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources