Biological consequences and advantages of asymmetric bacterial growth
- PMID: 23808335
- PMCID: PMC4001765
- DOI: 10.1146/annurev-micro-092412-155622
Biological consequences and advantages of asymmetric bacterial growth
Abstract
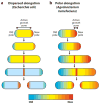
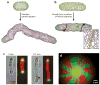
Asymmetries in cell growth and division occur in eukaryotes and prokaryotes alike. Even seemingly simple and morphologically symmetric cell division processes belie inherent underlying asymmetries in the composition of the resulting daughter cells. We consider the types of asymmetry that arise in various bacterial cell growth and division processes, which include both conditionally activated mechanisms and constitutive, hardwired aspects of bacterial life histories. Although asymmetry disposes some cells to the deleterious effects of aging, it may also benefit populations by efficiently purging accumulated damage and rejuvenating newborn cells. Asymmetries may also generate phenotypic variation required for successful exploitation of variable environments, even when extrinsic changes outpace the capacity of cells to sense and respond to challenges. We propose specific experimental approaches to further develop our understanding of the prevalence and the ultimate importance of asymmetric bacterial growth.
Figures



References
-
- Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol. 2007;64:938–52. - PubMed
-
- Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. Conducted experiments in Caulobacter crescentus to provide the first observations of bacterial aging. - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

