Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice
- PMID: 23808384
- PMCID: PMC3931116
- DOI: 10.1165/rcmb.2013-0070OC
Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice
Abstract
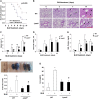
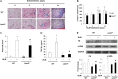
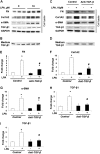
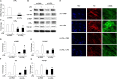
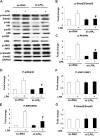
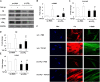
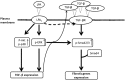
Idiopathic pulmonary fibrosis is a devastating disease characterized by alveolar epithelial cell injury, the accumulation of fibroblasts/myofibroblasts, and the deposition of extracellular matrix proteins. Lysophosphatidic acid (LPA) signaling through its G protein-coupled receptors is critical for its various biological functions. Recently, LPA and LPA receptor 1 were implicated in lung fibrogenesis. However, the role of other LPA receptors in fibrosis remains unclear. Here, we use a bleomycin-induced pulmonary fibrosis model to investigate the roles of LPA2 in pulmonary fibrogenesis. In the present study, we found that LPA2 knockout (Lpar2(-/-)) mice were protected against bleomycin-induced lung injury, fibrosis, and mortality, compared with wild-type control mice. Furthermore, LPA2 deficiency attenuated the bleomycin-induced expression of fibronectin (FN), α-smooth muscle actin (α-SMA), and collagen in lung tissue, as well as levels of IL-6, transforming growth factor-β (TGF-β), and total protein in bronchoalveolar lavage fluid. In human lung fibroblasts, the knockdown of LPA2 attenuated the LPA-induced expression of TGF-β1 and the differentiation of lung fibroblasts to myofibroblasts, resulting in the decreased expression of FN, α-SMA, and collagen, as well as decreased activation of extracellular regulated kinase 1/2, Akt, Smad3, and p38 mitogen-activated protein kinase. Moreover, the knockdown of LPA2 with small interfering RNA also mitigated the TGF-β1-induced differentiation of lung fibroblasts. In addition, LPA2 deficiency significantly attenuated the bleomycin-induced apoptosis of alveolar and bronchial epithelial cells in the mouse lung. Together, our data indicate that the knockdown of LPA2 attenuated bleomycin-induced lung injury and pulmonary fibrosis, and this may be related to an inhibition of the LPA-induced expression of TGF-β and the activation and differentiation of fibroblasts.
Figures
Similar articles
-
The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury.Am J Respir Cell Mol Biol. 2012 Mar;46(3):355-64. doi: 10.1165/rcmb.2010-0155OC. Epub 2011 Oct 20. Am J Respir Cell Mol Biol. 2012. PMID: 22021336 Free PMC article.
-
[Digoxin alleviates pulmonary fibrosis by regulating phosphatidylinositol-3-kinase/Akt signaling through inhibiting the activation of fibroblast: an in vivo and in vitro experiment].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Nov;34(11):1161-1166. doi: 10.3760/cma.j.cn121430-20220628-00508. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36567559 Chinese.
-
Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q).Am J Pathol. 2009 Apr;174(4):1264-79. doi: 10.2353/ajpath.2009.080160. Epub 2009 Jan 15. Am J Pathol. 2009. PMID: 19147812 Free PMC article.
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
-
CTHRC1: An Emerging Hallmark of Pathogenic Fibroblasts in Lung Fibrosis.Cells. 2024 May 30;13(11):946. doi: 10.3390/cells13110946. Cells. 2024. PMID: 38891078 Free PMC article. Review.
Cited by
-
Autotaxin and Endotoxin-Induced Acute Lung Injury.PLoS One. 2015 Jul 21;10(7):e0133619. doi: 10.1371/journal.pone.0133619. eCollection 2015. PLoS One. 2015. PMID: 26196781 Free PMC article.
-
Cellular and Molecular Control of Lipid Metabolism in Idiopathic Pulmonary Fibrosis: Clinical Application of the Lysophosphatidic Acid Pathway.Cells. 2023 Feb 8;12(4):548. doi: 10.3390/cells12040548. Cells. 2023. PMID: 36831215 Free PMC article. Review.
-
Autotaxin-LPA receptor axis in the pathogenesis of lung diseases.Int J Clin Exp Med. 2015 Oct 15;8(10):17117-22. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26770305 Free PMC article. Review.
-
Integrating inflammatory biomarker analysis and artificial-intelligence-enabled image-based profiling to identify drug targets for intestinal fibrosis.Cell Chem Biol. 2023 Sep 21;30(9):1169-1182.e8. doi: 10.1016/j.chembiol.2023.06.014. Epub 2023 Jul 11. Cell Chem Biol. 2023. PMID: 37437569 Free PMC article.
-
CREB-dependent LPA-induced signaling initiates a pro-fibrotic feedback loop between small airway basal cells and fibroblasts.Respir Res. 2021 Apr 1;22(1):97. doi: 10.1186/s12931-021-01677-0. Respir Res. 2021. PMID: 33794877 Free PMC article.
References
-
- King TE., Jr Update in pulmonary medicine. Ann Intern Med. 1998;129:806–812. - PubMed
-
- Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, II, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. - PubMed
-
- Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor–beta1–induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. - PubMed
-
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. - PubMed
-
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(Suppl. 6):286S–289S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

