Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice
- PMID: 23808551
- PMCID: PMC3753728
- DOI: 10.1089/hgtb.2013.076
Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice
Abstract
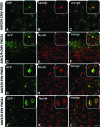
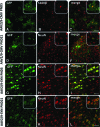
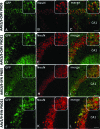
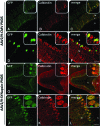
Several neurodevelopmental and neurodegenerative disorders affecting the central nervous system are potentially treatable via viral vector-mediated gene transfer. Adeno-associated viral (AAV) vectors have been used in clinical trials because of their desirable properties including a high degree of safety, efficacy, and stability. Major factors affecting tropism, expression level, and cell type specificity of AAV-mediated transgenes include encapsidation of different AAV serotypes, promoter selection, and the timing of vector administration. In this study, we evaluated the ability of single-stranded AAV2 vectors pseudotyped with viral capsids from serotype 9 (AAV2/9) to transduce the brain and target gene expression to specific cell types after intracerebroventricular injection into mice. Titer-matched AAV2/9 vectors encoding the enhanced green fluorescent protein (eGFP) reporter, driven by the cytomegalovirus (CMV) promoter, or the neuron-specific synapsin-1 promoter, were injected bilaterally into the lateral ventricles of C57/BL6 mice on postnatal day 5 (neonatal) or 21 (juvenile). Brain sections were analyzed 25 days after injection, using immunocytochemistry and confocal microscopy. eGFP immunohistochemistry after neonatal and juvenile administration of viral vectors revealed transduction throughout the brain including the striatum, hippocampus, cerebral cortex, and cerebellum, but with different patterns of cell-specific gene expression. eGFP expression was seen in astrocytes after treatment on postnatal day 5 with vectors carrying the CMV promoter, expanding the usefulness of AAVs for modeling and treating diseases involving glial cell pathology. In contrast, injection of AAV2/9-CMV-eGFP on postnatal day 21 resulted in preferential transduction of neurons. Administration of AAV2/9-eGFP with the synapsin-1 promoter on either postnatal day 5 or 21 resulted in widespread neuronal transduction. These results outline efficient methods and tools for gene delivery to the nervous system by direct, early postnatal administration of AAV vectors. Our findings highlight the importance of promoter selection and age of administration on the intensity, distribution, and cell type specificity of AAV transduction in the brain.
Figures

Similar articles
-
Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection.PLoS One. 2017 Dec 15;12(12):e0188830. doi: 10.1371/journal.pone.0188830. eCollection 2017. PLoS One. 2017. PMID: 29244806 Free PMC article.
-
Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain.Neuroscience. 2006;138(2):501-10. doi: 10.1016/j.neuroscience.2005.11.057. Epub 2006 Jan 18. Neuroscience. 2006. PMID: 16414198
-
Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain.PLoS One. 2013 Jun 25;8(6):e67680. doi: 10.1371/journal.pone.0067680. Print 2013. PLoS One. 2013. PMID: 23825679 Free PMC article.
-
Recombinant AAV-mediated gene delivery to the central nervous system.J Gene Med. 2004 Feb;6 Suppl 1:S212-22. doi: 10.1002/jgm.506. J Gene Med. 2004. PMID: 14978764 Review.
-
Tissue and cell-type-specific transduction using rAAV vectors in lung diseases.J Mol Med (Berl). 2021 Aug;99(8):1057-1071. doi: 10.1007/s00109-021-02086-y. Epub 2021 May 21. J Mol Med (Berl). 2021. PMID: 34021360 Review.
Cited by
-
Transduction Profile of the Marmoset Central Nervous System Using Adeno-Associated Virus Serotype 9 Vectors.Mol Neurobiol. 2017 Apr;54(3):1745-1758. doi: 10.1007/s12035-016-9777-6. Epub 2016 Feb 16. Mol Neurobiol. 2017. PMID: 26884266
-
Induction of Neuron-Specific Degradation of Coenzyme A Models Pantothenate Kinase-Associated Neurodegeneration by Reducing Motor Coordination in Mice.PLoS One. 2015 Jun 8;10(6):e0130013. doi: 10.1371/journal.pone.0130013. eCollection 2015. PLoS One. 2015. PMID: 26052948 Free PMC article.
-
In Vivo Expression of Reprogramming Factor OCT4 Ameliorates Myelination Deficits and Induces Striatal Neuroprotection in Huntington's Disease.Genes (Basel). 2021 May 10;12(5):712. doi: 10.3390/genes12050712. Genes (Basel). 2021. PMID: 34068799 Free PMC article.
-
Survival benefit and phenotypic improvement by hamartin gene therapy in a tuberous sclerosis mouse brain model.Neurobiol Dis. 2015 Oct;82:22-31. doi: 10.1016/j.nbd.2015.04.018. Epub 2015 May 24. Neurobiol Dis. 2015. PMID: 26019056 Free PMC article.
-
Impacts of CACNB4 overexpression on dendritic spine density in both sexes and relevance to schizophrenia.Transl Psychiatry. 2024 Dec 4;14(1):484. doi: 10.1038/s41398-024-03181-7. Transl Psychiatry. 2024. PMID: 39632796 Free PMC article.
References
-
- Bockstael O. Foust K. Kaspar B. Tenenbaum L. Recombinant AAV delivery to the central nervous system. Methods Mol. Biol. 2011;807:159–177. - PubMed
-
- Cearley C.N. Wolfe J.H. Transduction characteristics of adeno-associated virus vectors expressing Cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006;13:528–537. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

