Crystal structure of a human prion protein fragment reveals a motif for oligomer formation
- PMID: 23808589
- PMCID: PMC3766960
- DOI: 10.1021/ja403001q
Crystal structure of a human prion protein fragment reveals a motif for oligomer formation
Abstract
The structural transition of the prion protein from α-helical- to β-sheet-rich underlies its conversion into infectious and disease-associated isoforms. Here we describe the crystal structure of a fragment from human prion protein consisting of the disulfide-bond-linked portions of helices 2 and 3. Instead of forming a pair-of-sheets steric zipper structure characteristic of amyloid fibers, this fragment crystallized into a β-sheet-rich assembly of hexameric oligomers. This study reveals a never before observed structural motif for ordered protein aggregates and suggests a possible mechanism for self-propagation of misfolded conformations by such nonamyloid oligomers.
Figures
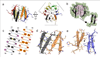

References
-
- Haass C, Selkoe DJ. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Nature. 2011;470:540–542. - PubMed
-
- Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJW, McFarlane HT, Madsen AØ, Riekel C, Eisenberg D. Nature. 2007;447:453–457. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

