Arsenic binding to proteins
- PMID: 23808632
- PMCID: PMC3797521
- DOI: 10.1021/cr300015c
Arsenic binding to proteins
Figures


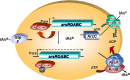
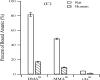
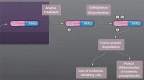
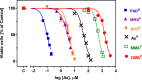
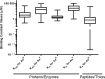
References
-
- Cullen W. R.Is Arsenic an Aphrodisiac? The Sociochemistry of an Element; RSC Publishing: Cambridge, U.K., 2008; 412 pp.
-
- Oremland R. S.; Stolz J. F. Science 2003, 300, 939. - PubMed
-
- Nordstrom D. K. Science 2002, 296, 2143. - PubMed
-
- Jain C. K.; Ali I. Water Res. 2000, 34, 4304.
-
- Bhattacharya P.; Welch A. H.; Stollenwerk K. G.; McLaughlin M. J.; Bundschuh J.; Panaullah G. Sci. Total Environ. 2007, 379, 109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

