pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators
- PMID: 23808707
- PMCID: PMC3773053
- DOI: 10.1111/head.12152
pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators
Abstract
Background: Prior studies have shown that decreased meningeal pH activates dural afferents via opening of acid-sensing ion channels (ASICs), suggesting one pathophysiological mechanism for the generation of headaches. The studies described here further examined the ASIC subtype mediating pH-induced dural-afferent activation and examined whether sensitization influences pH responses.
Objective: Given the potential importance of meningeal mast cells to headache, the goal of this study was to evaluate dural afferent responses to pH following sensitization with mast cell mediators.
Methods: Cutaneous allodynia was measured in rats following stimulation of the dura with decreased pH alone or in combination with mast cell mediators. Trigeminal ganglion neurons retrogradely labeled from the dura were stained with an ASIC3 antibody using immunohistochemistry. Current and action potentials evoked by changes in pH alone or in combination with mast cell mediators were measured in retrogradely labeled dural afferents using patch-clamp electrophysiology.
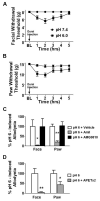
Results: pH-sensitive dural afferents generated currents in response to the ASIC3 activator 2-guanidine-4-methylquinazoline (GMQ), approximately 80% of these neurons express ASIC3 protein, and pH-evoked behavioral responses were inhibited by the ASIC3 blocker APETx2. Following exposure to mast cell mediators, dural afferents exhibited increased pH-evoked excitability, and cutaneous allodynia was observed at higher pH than with pH stimuli alone.
Conclusions: These data indicate that the predominant ASIC subtype responding to decreased meningeal pH is ASIC3. Additionally, they demonstrate that in the presence of inflammation, dural afferents respond to even smaller decreases in pH providing further support for the ability of small pH changes within the meninges to initiate afferent input leading to headache.
Keywords: ASIC3; dural afferent; meninges.
© 2013 American Headache Society.
Figures





Comment in
-
ASIC3: first the heartache, now a migraine!Headache. 2013 Sep;53(8):1204-6. doi: 10.1111/head.12170. Headache. 2013. PMID: 24020955 No abstract available.
References
-
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–270. - PubMed
-
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. - PubMed
-
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

