Top 10 plant-parasitic nematodes in molecular plant pathology
- PMID: 23809086
- PMCID: PMC6638764
- DOI: 10.1111/mpp.12057
Top 10 plant-parasitic nematodes in molecular plant pathology
Abstract
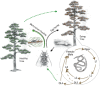
The aim of this review was to undertake a survey of researchers working with plant-parasitic nematodes in order to determine a 'top 10' list of these pathogens based on scientific and economic importance. Any such list will not be definitive as economic importance will vary depending on the region of the world in which a researcher is based. However, care was taken to include researchers from as many parts of the world as possible when carrying out the survey. The top 10 list emerging from the survey is composed of: (1) root-knot nematodes (Meloidogyne spp.); (2) cyst nematodes (Heterodera and Globodera spp.); (3) root lesion nematodes (Pratylenchus spp.); (4) the burrowing nematode Radopholus similis; (5) Ditylenchus dipsaci; (6) the pine wilt nematode Bursaphelenchus xylophilus; (7) the reniform nematode Rotylenchulus reniformis; (8) Xiphinema index (the only virus vector nematode to make the list); (9) Nacobbus aberrans; and (10) Aphelenchoides besseyi. The biology of each nematode (or nematode group) is reviewed briefly.
© 2013 BSPP AND JOHN WILEY & SONS LTD.
Figures












References
-
- Abad, P. , Gouzy, J. , Aury, J.‐M. , Castagnone‐Sereno, P. , Danchin, E.G.J. , Deleury, E. , Perfus‐Barbeoch, L. , Anthouard, V. , Artiguenave, F. , Blok, V.C. , Caillaud, M.‐C. , Coutinho, P.M. , Dasilva, C. , De Luca, F. , Deau, F. , Esquibet, M. , Flutre, T. , Goldstone, J.V. , Hamamouch, N. , Hewezi, T. , Jaillon, O. , Jubin, C. , Leonetti, P. , Magliano, M. , Maier, T.R. , Markov, G.V. , McVeigh, P. , Pesole, G. , Poulain, J. , Robinson‐Rechavi, M. , Sallet, E. , Segurens, B. , Steinbach, D. , Tytgat, T. , Ugarte, E. , van Ghelder, C. , Veronico, P. , Baum, T.J. , Blaxter, M. , Bleve‐Zacheo, T. , Davis, E.L. , Ewbank, J.J. , Favery, B. , Grenier, E. , Henrissat, B. , Jones, J.T. , Laudet, V. , Maule, A.G. , Quesneville, H. , Rosso, M.N. , Schiex, T. , Smant, G. , Weissenbach, J. and Wincke, P. (2008) Genome sequence of the metazoan plant‐parasitic nematode Meloidogyne incognita . Nat. Biotechnol. 26, 909–915. - PubMed
-
- Al‐Banna, L. , Williamson, V. and Gardner, S.L. (1997) Phylogenetic analysis of nematodes of the genus Pratylenchus using nuclear 26S rDNA. Mol. Phylogenet. Evol. 7, 94–102. - PubMed
-
- Anthoine, G. and Mugniéry, D. (2005) Variability of the ITS rDNA and identification of Nacobbus aberrans (Thorne, 1935) Thorne and Allen, 1944 (Nematoda: Pratylenchidae) by rDNA amplification. Nematology, 7, 503–516.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

