Structure-function and regulation of ADAMTS-13 protease
- PMID: 23809107
- PMCID: PMC3713533
- DOI: 10.1111/jth.12221
Structure-function and regulation of ADAMTS-13 protease
Abstract
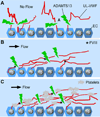
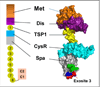
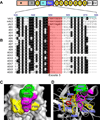
ADAMTS-13, a plasma reprolysin-like metalloprotease, cleaves von Willebrand factor (VWF). Severe deficiency of plasma ADAMTS-13 activity results in thrombotic thrombocytopenic purpura (TTP), while mild to moderate deficiencies of plasma ADAMTS-13 activity are emerging risk factors for developing myocardial and cerebral infarction, pre-eclampsia, and malignant malaria. Moreover, Adamts13(-/-) mice develop more severe inflammatory responses, leading to increased ischemia/perfusion injury and formation of atherosclerosis. Structure-function studies demonstrate that the N-terminal portion of ADAMTS-13 (MDTCS) is necessary and sufficient for proteolytic cleavage of VWF under various conditions and attenuation of arterial/venous thrombosis after oxidative injury. The more distal portion of ADAMTS-13 (TSP1 2-8 repeats and CUB domains) may function as a disulfide bond reductase to prevent an elongation of ultra-large VWF strings on activated endothelial cells and inhibit platelet adhesion/aggregation on collagen surface under flow. Remarkably, the proteolytic cleavage of VWF by ADAMTS-13 is accelerated by FVIII and platelets under fluid shear stress. A disruption of the interactions between FVIII (or platelet glycoprotein 1bα) and VWF dramatically impairs ADAMTS-13-dependent proteolysis of VWF in vitro and in vivo. These results suggest that FVIII and platelets may be physiological cofactors regulating VWF proteolysis. Finally, the structure-function and autoantibody mapping studies allow us to identify an ADAMTS-13 variant with increased specific activity but reduced inhibition by autoantibodies in patients with acquired TTP. Together, these findings provide novel insight into the mechanism of VWF proteolysis and tools for the therapy of acquired TTP and perhaps other arterial thrombotic disorders.
Keywords: ADAMTS13 protein; animals; arterial thrombosis; autoimmune diseases; factor VIII; human; thrombotic microangiopathies; von Willebrand factor (445-733).
© 2013 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Figures

Similar articles
-
Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner.Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):397-407. doi: 10.1161/ATVBAHA.113.302547. Epub 2013 Dec 19. Arterioscler Thromb Vasc Biol. 2014. PMID: 24357063 Free PMC article.
-
[ADAMTS13-Mediated Proteolytic Cleavage of Unusually Large von Willebrand Factor Polymers on Endothelial Cells in the Absence of Fluid Shear Stress].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024 Apr;32(2):532-540. doi: 10.19746/j.cnki.issn.1009-2137.2024.02.032. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024. PMID: 38660863 Chinese.
-
ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor.J Biol Chem. 2003 Aug 8;278(32):29633-9. doi: 10.1074/jbc.M301385200. Epub 2003 May 29. J Biol Chem. 2003. PMID: 12775718
-
Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays.Semin Thromb Hemost. 2005 Dec;31(6):659-72. doi: 10.1055/s-2005-925472. Semin Thromb Hemost. 2005. PMID: 16388417 Free PMC article. Review.
-
The role of ADAMT-13 in platelet adhesion in flow: methods for diagnosis of thrombotic thrombocytopenic purpura.Pathophysiol Haemost Thromb. 2006;35(1-2):98-102. doi: 10.1159/000093550. Pathophysiol Haemost Thromb. 2006. PMID: 16855353 Review.
Cited by
-
Determinants of versican-V1 proteoglycan processing by the metalloproteinase ADAMTS5.J Biol Chem. 2014 Oct 3;289(40):27859-73. doi: 10.1074/jbc.M114.573287. Epub 2014 Aug 13. J Biol Chem. 2014. PMID: 25122765 Free PMC article.
-
Peptide-Based Inhibitors of ADAM and ADAMTS Metalloproteinases.Front Mol Biosci. 2021 Jul 21;8:703715. doi: 10.3389/fmolb.2021.703715. eCollection 2021. Front Mol Biosci. 2021. PMID: 34368231 Free PMC article. Review.
-
Low ADAMTS-13 predicts adverse outcomes in hospitalized patients with suspected heparin-induced thrombocytopenia.Res Pract Thromb Haemost. 2021 Sep 16;5(6):e12581. doi: 10.1002/rth2.12581. eCollection 2021 Aug. Res Pract Thromb Haemost. 2021. PMID: 34553121 Free PMC article.
-
Increased von Willebrand factor over decreased ADAMTS-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer.J Clin Lab Anal. 2018 Jan;32(1):e22219. doi: 10.1002/jcla.22219. Epub 2017 Apr 4. J Clin Lab Anal. 2018. PMID: 28374895 Free PMC article.
-
[New progress in clinical significance of ADAMTS13].Zhonghua Xue Ye Xue Za Zhi. 2017 Oct 14;38(10):903-906. doi: 10.3760/cma.j.issn.0253-2727.2017.10.018. Zhonghua Xue Ye Xue Za Zhi. 2017. PMID: 29166749 Free PMC article. Chinese. No abstract available.
References
-
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. - PubMed
-
- Zheng XL, Chung D, Takayama T, Majerus E, Sadler J, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. - PubMed
-
- Sadler JE. von Willebrand factor. J Biol Chem. 1991;266:22777–22780. - PubMed
-
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

