K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter
- PMID: 23809161
- PMCID: PMC3730833
- DOI: 10.1016/j.immuni.2013.05.016
K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter
Abstract
The NLRP3 inflammasome is an important component of the innate immune system. However, its mechanism of activation remains largely unknown. We show that NLRP3 activators including bacterial pore-forming toxins, nigericin, ATP, and particulate matter caused mitochondrial perturbation or the opening of a large membrane pore, but this was not required for NLRP3 activation. Furthermore, reactive oxygen species generation or a change in cell volume was not necessary for NLRP3 activation. Instead, the only common activity induced by all NLRP3 agonists was the permeation of the cell membrane to K⁺ and Na⁺. Notably, reduction of the intracellular K⁺ concentration was sufficient to activate NLRP3, whereas an increase in intracellular Na⁺ modulated but was not strictly required for inflammasome activation. These results provide a unifying model for the activation of the NLRP3 inflammasome in which a drop in cytosolic K⁺ is the common step that is necessary and sufficient for caspase-1 activation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no financial conflict of interest.
Figures
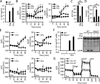
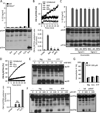
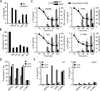
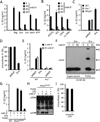

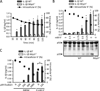
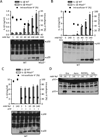
Comment in
-
K⁺ drops tilt the NLRP3 inflammasome.Immunity. 2013 Jun 27;38(6):1085-8. doi: 10.1016/j.immuni.2013.06.001. Immunity. 2013. PMID: 23809157
References
-
- Allam R, Darisipudi MN, Rupanagudi KV, Lichtnekert J, Tschopp J, Anders HJ. Cutting edge: cyclic polypeptide and aminoglycoside antibiotics trigger IL-1beta secretion by activating the NLRP3 inflammasome. J Immunol. 2011;186:2714–2718. - PubMed
-
- Andersen OS, Koeppe RE, Roux B. Gramicidin channels. IEEE Transactions on Nanobioscience. 2005;4:10–20. - PubMed
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5 R25 GM086262/GM/NIGMS NIH HHS/United States
- R56 AI063331/AI/NIAID NIH HHS/United States
- DK089503/DK/NIDDK NIH HHS/United States
- AI06333/AI/NIAID NIH HHS/United States
- R01 DK091191/DK/NIDDK NIH HHS/United States
- R37 AI063331/AI/NIAID NIH HHS/United States
- AR059688/AR/NIAMS NIH HHS/United States
- R25 GM086262/GM/NIGMS NIH HHS/United States
- R01 AR059688/AR/NIAMS NIH HHS/United States
- R01 AI063331/AI/NIAID NIH HHS/United States
- R01 AI064748/AI/NIAID NIH HHS/United States
- DK091191/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

