MicroRNA networks regulate development of brown adipocytes
- PMID: 23809233
- PMCID: PMC3979327
- DOI: 10.1016/j.tem.2013.05.002
MicroRNA networks regulate development of brown adipocytes
Abstract
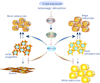
Brown adipose tissue (BAT) is specialized for heat generation and energy expenditure as a defense against cold and obesity; in both humans and mice increased amounts of BAT are associated with a lean phenotype and resistance to development of the metabolic syndrome and its complications. Here we summarize recent research showing that several BAT-expressed microRNAs (miRNAs) play important roles in regulating differentiation and metabolism of brown and beige adipocytes; we discuss the key mRNA targets downregulated by these miRNAs and show how these miRNAs affect directly or indirectly transcription factors important for BAT development. We suggest that these miRNAs could be part of novel therapeutics to increase BAT in humans.
Keywords: adipocyte; adipogenesis; beige; brown; microRNA.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue (Translated from eng) Nature. 1994;372(6505):425–432. (in eng). - PubMed
-
- Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice (Translated from eng) Science. 1995;269(5223):540–543. (in eng). - PubMed
-
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes (Translated from eng) J Biol Chem. 1995;270(45):26746–26749. (in eng). - PubMed
-
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity (Translated from eng) J Biol Chem. 1996;271(18):10697–10703. (in eng). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

