Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways
- PMID: 23809253
- PMCID: PMC4156008
- DOI: 10.1111/febs.12415
Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways
Abstract
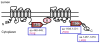
Defining the significant checkpoints in cystic fibrosis transmembrane conductance regulator (CFTR) biogenesis should identify targets for therapeutic intervention with CFTR folding mutants such as F508del. Although the role of ubiquitylation and the ubiquitin proteasome system is well established in the degradation of this common CFTR mutant, the part played by SUMOylation is a novel aspect of CFTR biogenesis/quality control. We identified this post-translational modification of CFTR as resulting from its interaction with small heat shock proteins (Hsps), which were found to selectively facilitate the degradation of F508del through a physical interaction with the SUMO (small ubiquitin-like modifier) E2 enzyme, Ubc9. Hsp27 promoted the SUMOylation of mutant CFTR by the SUMO-2 paralogue, which can form poly-chains. Poly-SUMO chains are then recognized by the SUMO-targeted ubiquitin ligase, RNF4, which elicited F508del degradation in a Hsp27-dependent manner. This work identifies a sequential connection between the SUMO and ubiquitin modifications of the CFTR mutant: Hsp27-mediated SUMO-2 modification, followed by ubiquitylation via RNF4 and degradation of the mutant via the proteasome. Other examples of the intricate cross-talk between the SUMO and ubiquitin pathways are discussed with reference to other substrates; many of these are competitive and lead to different outcomes. It is reasonable to anticipate that further research on SUMO-ubiquitin pathway interactions will identify additional layers of complexity in the process of CFTR biogenesis and quality control.
Keywords: Hsp27; STUbL; cystic fibrosis transmembrane conductance regulator; degradation; endoplasmic reticulum associated; proteasome; quality control; small heat shock proteins; small ubiquitin-like modifier; ubiquitin.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures
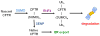
References
-
- Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–55. - PubMed
-
- Kerem E, Kerem B. The relationship between genotype and phenotype in cystic fibrosis. Curr Opin Pulm Med. 1995;1:450–6. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. - PubMed
-
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–34. - PubMed
-
- Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

