Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring
- PMID: 23809871
- PMCID: PMC3726366
- DOI: 10.1186/1477-9560-11-10
Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring
Abstract
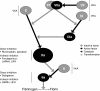
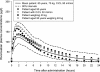
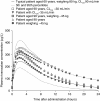
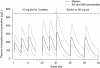
Unlike traditional anticoagulants, the more recently developed agents rivaroxaban, dabigatran and apixaban target specific factors in the coagulation cascade to attenuate thrombosis. Rivaroxaban and apixaban directly inhibit Factor Xa, whereas dabigatran directly inhibits thrombin. All three drugs exhibit predictable pharmacokinetic and pharmacodynamic characteristics that allow for fixed oral doses in a variety of settings. The population pharmacokinetics of rivaroxaban, and also dabigatran, have been evaluated in a series of models using patient data from phase II and III clinical studies. These models point towards a consistent pharmacokinetic and pharmacodynamic profile, even when extreme demographic factors are taken into account, meaning that doses rarely need to be adjusted. The exception is in certain patients with renal impairment, for whom pharmacokinetic modelling provided the rationale for reduced doses as part of some regimens. Although not routinely required, the ability to measure plasma concentrations of these agents could be advantageous in emergency situations, such as overdose. Specific pharmacokinetic and pharmacodynamic characteristics must be taken into account when selecting an appropriate assay for monitoring. The anti-Factor Xa chromogenic assays now available are likely to provide the most appropriate means of determining plasma concentrations of rivaroxaban and apixaban, and specific assays for dabigatran are in development.
Figures
References
-
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S. doi: 10.1378/chest.11-2292. - DOI - PMC - PubMed
-
- Bayer Pharma AG. Xarelto® (rivaroxaban) summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- Janssen Pharmaceuticals Inc. Xarelto® (rivaroxaban) prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf.
-
- Bristol-Myers S, Pfizer EEIG. Eliquis® (apixaban) summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
LinkOut - more resources
Full Text Sources
Other Literature Sources

